This is "An Overview of OChem 1 Lab", from the book 32 Weeks of OChem (v. 1.0).
An Overview of OChem 1 Lab
Learning Objectives
- To know and distinguish between the three sorts of laboratory work you will encounter in OChem Lab: synthesis, workup and characterization.
- To be able to distingish a reaction equation from a reaction mechanism.
- To memorize the names of six reaction mechanisms.
- To memorize the names of seven elementary steps.
- To know the names of some different types of workup procedures.
- To know the names of some different characterization techniques.
- To know how the points are distributed for the lab portion of the course.
Organic chemistry laboratory can be a little overwhelming. It is helpful to see how what your are doing fits into a big picture. In the big picture, there are three main activities that take place in the lab:
- Synthesis
- Workup
- Characterization
Synthesis
Synthesis in its simplest definition is the act of chemical change. Reactants are combined to make products. There's more to the story than just following a recipe.
Figure 0.1 (Lab) The Easy-Bake Oven

By Bradross63 (Own work) [CC BY-SA 4.0 (http://creativecommons.org/licenses/by-sa/4.0)], via Wikimedia Commons
Successful organic syntheses require thoughtful strategy and masterful technique. Organic synthesis is not an art, neither does it depend on luck nor magic. Organic synthesis is a practical science, chock full of reason, explanation and purpose. Lab work is a skill that takes practice. You are guaranteed to have failures. Learn to roll with the punches and follow your hunches. The best practice for organic synthesis is to start with a reaction mechanism. Although in general chemistry we put great emphasis on writing balanced equations, in organic chemistry we put a much higher priority on the reaction mechanism. Yes, both equations and mechanisms have arrows that point from left to right or sometimes both directions and this can be a point of confusion. But remember, a chemical equation succinctly describes the overall chemical change. A reaction mechanism is a sequence of elementary steps that add up to the overall equation. Recall a reaction mechanism can never be proved correct, only proposed. Keep in mind the reactions we will be doing in our laboratory have well-accepted mechanisms. If we have observations contrary to an accepted mechanism, we will become world-famous. Over the course of the first semester we will perform reactions with 6 different mechanisms. Some mechanisms are only one elementary step. Other mechanisms are a sequence of elementary steps. In Table 0.1 (Lab) is a list of the mechanisms for this semester and their elementary steps.
Table 0.1 (Lab) Mechanisms and Elementary Steps for OChem 1
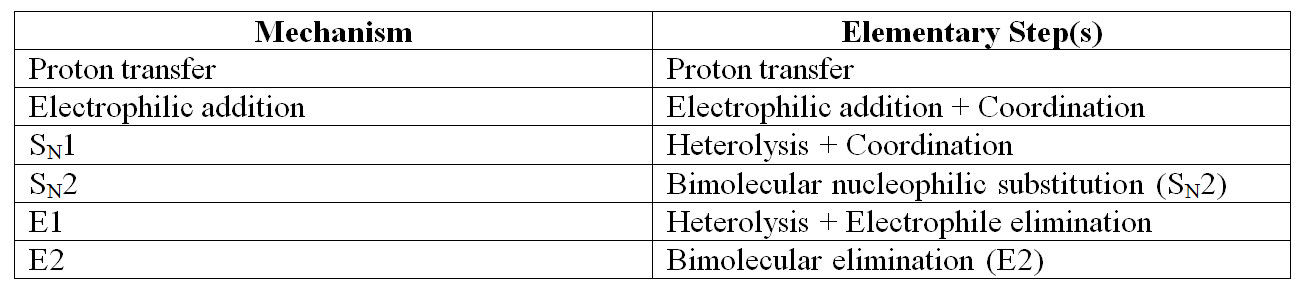
At this point, the names of the mechanisms and the elementary steps are just words and acronyms that have no meaning for you. Good! Memorize these words and acronyms now. Their meaning will be abundantly clear over the course of the semester. Doing this little bit of memorization now gives you a toe hold as you encounter these mechanisms and steps throughout the course.
Workup
If you used your Easy-Bake Oven to bake a cake, you would put the ingredients together, bake and then have a delicious cake at the end. Organic chemistry is usually not so simple. You put the ingredients together, sometimes bake, sometimes not, but what you end up with is not the final product. Instead, what you have is a crude mixture of the desired product, unreacted reagents, side products and solvent. Your task in workup is to isolate the desired product from this crude reaction mixture. If your product is a solid and the reactants, solvents and side products are all liquids or gases, you can easily isolate your product by filtration. More often, the separation is a little more involved than a simple filtration. Although the theory of separation and development of techniques fall in the realm of analytical chemistry, organic chemists employ a variety of separation techniques:
- Distillation
- Solvent Extraction
- Crystallization/Recrystallization
Since proton transfer reactions are easily reversible, one frequently encounters a proton transfer step in the workup to facilitate separation. Proton transfer steps can make the line between Synthesis and Workup a little blurry.
Characterization
Once a product is synthesized and purified, it's time to do some experiments to see what it is exactly that you made. Such experiments are called characterization. Physical and chemical tests can be performed on your sample and the results can sometimes be compared to literature values or interpreted on their own. Some of the characterization tests we will perform include:
- Melting point
- Chemical tests including AgNO3 in EtOH, NaI in Acetone, Br2 in dichloromethane
- UV-Vis absorption
- FTIR
- Polarimetry
- Thin Layer Chromatography (TLC)
- Gas Chromatography (GC)
Other characterization techniques require instrumentation beyond our current resources; however, these characterization techniques will be thoroughly discussed in your lab experience and you will be well prepared to interpret the following experimental results:
- Mass spectrometry
- 1H-NMR
- 13C-NMR
Scoring
Lab accounts for 20% of your course grade. The total number of lab points possible is 1000.
Below is a schedule of activities, their associated scores and links to the assignments. Navigating to the assignment with the link will take you to the assignment's procedure and its scoring rubric.
Week 1 (8/21/19)
- Introduction and Orientation to Organic Chemistry Lab
- Pre-lab questions and discussion for "Titration of an Organic Acid" (30 points)
- Perform "Titration of an Organic Acid" (30 points)
- Spectroscopic determination of the molecular formula of a hydrocarbon
Week 2 (8/28/19)
- Spectroscopic identification of an unknown set #1 (10 points)
- Presentation of Titration of an Organic Acid (30 points)
- Post-lab questions for "Titration of an Organic Acid" (10 points)
- Pre-lab questions and discussion for "Acid-Base Extraction" (10 points)
- Spectroscopic detection of unsaturation in a hydrocarbon
Week 3 (9/4/19)
- Perform "Acid-Base Extraction" (40 points)
- Spectroscopic identification of an unknown set #2 (25 points)
Week 4 (9/11/19)
- Presentation of "Acid-Base Extraction" (40 points)
- Post-lab questions for "Acid-Base Extraction" (10 points)
- Pre-lab questions and discussion for "Acid-Catalyzed Hydrolysis of Sucrose" (10 points)
- Spectroscopic detection of oxygen
Week 5 (9/18/19)
- Perform "Acid-Catalyzed Hydrolysis of Sucrose" (40 points)
- Spectroscopic identification of an unknown set #3 (25 points)
Week 6 (9/25/19)
- Presentation of "Acid-Catalyzed Hydrolysis of Sucrose" (40 points)
- Post-lab questions for "Acid-Catalyzed Hydrolysis of Sucrose" (10 points)
- Pre-lab questions and discussion for "The Addition of HBr to Cyclohexene" (10 points)
- Spectroscopic detection of nitrogen
Week 7 (10/2/19)
- Perform "The Addition of HBr to Cyclohexene" (40 points)
- Spectroscopic identification of an unknown set #4 (25 points)
Week 8 (10/9/19)
- Presentation of "The Addition of HBr to Cyclohexene" (40 points)
- Post-lab questions for "The Addition of HBr to Cyclohexene" (10 points)
- Pre-lab questions and discussion for "Synthesis of Tert-Pentyl Chloride" (10 points)
- Spectroscopic detection of chlorine and bromine
Week 9 (10/16/19)
- Perform "Synthesis of Tert-Pentyl Chloride" (40 points)
- Spectroscopic identification of an unknown set #5 (25 points)
Week 10 (10/23/19)
- Presentation of "Synthesis of Tert-Pentyl Chloride" (40 points)
- Post-lab questions for "Synthesis of Tert-Pentyl Chloride" (10 points)
- Pre-lab questions and discussion for "Synthesis of ortho-Formylphenoxyacetic Acid" (10 points)
- Spectroscopic detection of a benzene ring
Week 11 (10/30/19)
- Perform "Synthesis of ortho-Formylphenoxyacetic Acid" (40 points)
- Spectroscopic identification of an unknown set #6 (25 points)
Week 12 (11/6/19)
- Presentation of "Synthesis of ortho-Formylphenoxyacetic Acid" (40 points)
- Post-lab questions for "Synthesis of ortho-Formylphenoxyacetic Acid" (10 points)
- Pre-lab questions and discussion for "Synthesis of Cyclohexene" (10 points)
- Spectroscopic discernment of substitution patterns of alkenes
Week 13 (11/13/19)
- Perform "Synthesis of Cyclohexene" (40 points)
- Spectroscopic identification of an unknown set #7 (25 points)
Week 14 (11/20/19)
- Presentation of "Synthesis of Cyclohexene" (40 points)
- Post-lab questions for "Synthesis of Cyclohexene" (10 points)
- Pre-lab questions and discussion for "Dehydration of 2-methylcyclohexanol" (10 points)
- 1H NMR spin-spin splitting: an introduction
Week 15 (11/27/19)
- Perform "Dehydration of 2-methylcyclohexanol" (40 points)
- Spectroscopic identification of an unknown set #8 (25 points)
Week 16 (12/4/19)
- Presentation of "Dehydration of 2-methylcyclohexanol" (65 points)
- MS fragmentaion: alpha-cleavage

