This is "Spectroscopic Detection of Chlorine and Bromine in Organic Compounds" from the book 32 Weeks of OChem (v. 1.0).
Spectroscopic Detection of Chlorine and Bromine in Organic Compounds

A U.S. soldier is demonstrating DDT-hand spraying equipment while applying the insecticide.
The use of DDT increased enormously on a worldwide basis after World War II, because of its effectiveness against the mosquito that spreads malaria and lice that carry typhus. The World Health Organization claims that the use of DDT saved 25 million lives.
During World War I typhus caused three million deaths in Russia and more in Poland and Romania. De-lousing stations were established for troops on the Western front but the disease ravaged the armies of the Eastern front, with over 150,000 dying in Serbia alone. Fatalities were generally between 10 to 40 percent of those infected, and the disease was a major cause of death for those nursing the sick. Between 1918 and 1922 typhus caused at least 3 million deaths out of 20–30 million cases. In Russia after World War I, during the civil war between the White and Red armies, typhus killed three million, largely civilians. Even larger epidemics in the post-war chaos of Europe were only averted by the widespread use of the newly discovered DDT to kill the lice on millions of refugees and displaced persons.By Image and description Content Providers(s): CDC [Public domain], via Wikimedia Commons
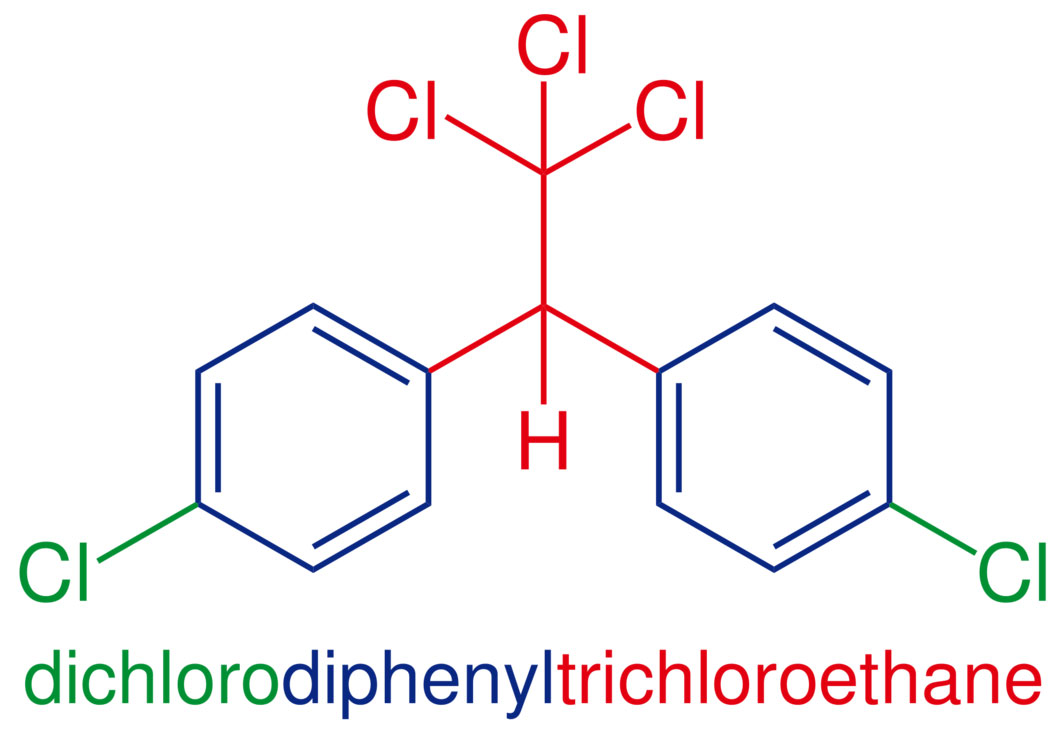
By Leyo (Own work) [Public domain], via Wikimedia Commons
Learning Objectives
- To interpret a mass spectrum of a compound which may contain chlorine or bromine.
- To know characteristic IR bands for compounds containing chlorine or bromine.
- To be able to associate 13C and 1H NMR chemical shift information with the presence of chlorine or bromine atoms in a compound.
Way back in general chemistry we learned the valence of a halogen is one. Meaning a chlorine or a bromine atom forms one bond in neutral compounds.
MS
The monovalent nature of of any halogen means it replaces an H in our molecular formula. The halogen atoms conveniently have odd masses. This means our nitrogen rule holds even in the presence of any number of halogen atoms. The molecular ion has an even numbered mass except for the cases of the presence of an odd number of nitrogens. Recall, the r+db formula.
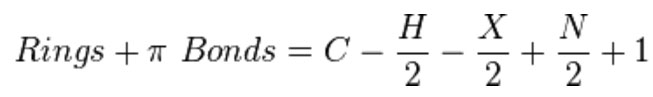
There is however a striking feature that is remarkably different when it comes to the mass spectrum of a compound with chlorine or bromine. That feature is the M+2 peak. Chlorine comes in two flavors, 35 amu and 37 amu. Bromine comes in two isotopic flavors, 79 amu and 81 amu. And the abundance of the heavy isotopes is ginormous compared to paltry 13C/12C (1.1%) or 18O/16O (0.2%) abundances.
37Cl/35Cl = 31.9%
81Br/79Br = 97.3%
This makes the presence of just one chlorine or bromine atom in the spectrum rather obvious. Consider the spectra below.
Figure 5.1 (Spec) Infrared Spectrum of a Chloroalkane
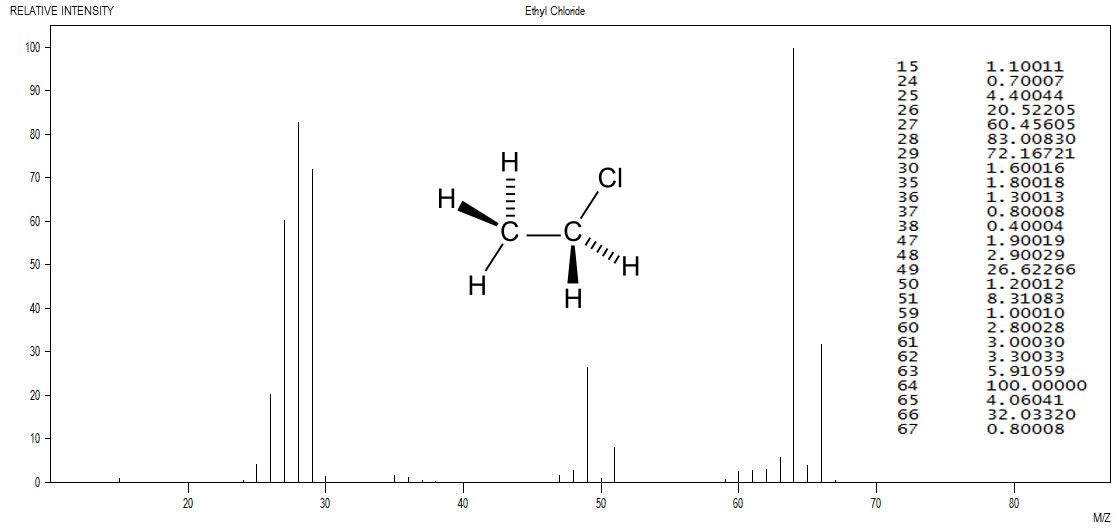
The M+2 peak is between a quarter and a third the intensity as the M+ peak. Such a pattern is a clear indication of the presence of a single chlorine atom. Data from the NIST was plotted with the JDXview program. The data table, also from the NIST was overlaid upon the spectrum along with the structure from Wikipedia.
Figure 5.2 (Spec) Infrared Spectrum of an Bromoalkane
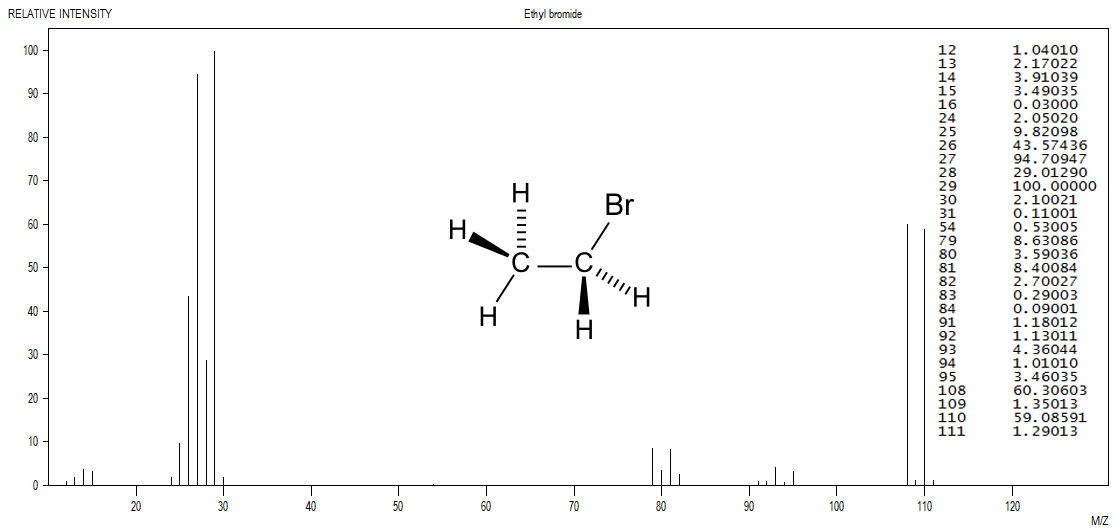
The M+ peak is nearly the same intensity as the M+2 peak. Such a pattern is a clear indication of the presence of a single bromine atom. Data from the NIST was plotted with the JDXview program. The data table, also from the NIST was overlaid upon the spectrum along with the structure from Wikipedia.
If more than one chlorine and/or bromine is present, the parent ion peak group gets more complex, though the possible patterns certainly remain distinctive. A very cool JavaScript program from T. W. Shattuck at Colby College is pasted below:




