This is "Dehydration of Cyclohexanol", from the book 32 Weeks of OChem (v. 1.0).
Dehydration of Cyclohexanol
Learning Objectives
- To know and write the mechanism for acid catalyzed E1 dehydration of an alcohol.
- To be able to use a Hickman condenser.
- To be able to efficiently use a drying agent.
- To perform an FTIR and use the spectrum as characterization of a product.
Figure 11.1 (Lab) Dehydration of Cyclohexanol
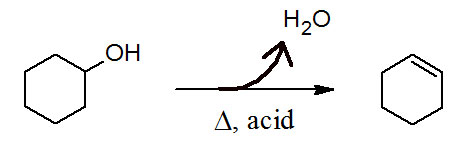
Secondary alcohols undergo E1 reactions to form alkenes. The most plausible mechanism starts with a proton transfer step, followed by a rate-limiting heterolysis step and wraps up with an electrophile elimination step regenerating the acid catalyst.
Weigh about 4 grams of cyclohexanol into a 10-mL round bottom flask. The rest of the procedure takes place in the hood. Cyclohexene is stinky and the concentrated acids are dangerously corrosive to human tissue. Wear appropriate PPE. Nest the RB flask into an aluminum heating block on a stirring hotplate. Add a stir bar and begin slowly stirring the cyclohexanol. Hold the RB upright with a clamp and ring stand. Use a calibrated disposable transfer pipet to add 1.0 mL of 85% H3PO4 and sixteen drops of concentrated H2SO4. Attach a Hickman condenser with a capped side port to the RB flask and secure the clamp. Attach the air reflux condenser above the Hickman. Suspend a thermometer such that the bulb hangs below the Hickman collection bowl. Start heating with the hotplate set around 2-3 to achieve a reflux. The temperature on the thermometer will rise to between 90-95 °C and the cyclohexene / water mixture will begin filling the Hickman bowl. A good yield might overflow the Hickman bowl, so it would be a good idea to remove some of the product mixture as the bowl fills. Use a glass tranfer pipet through the side port of the Hickman condenser. Put the distillate into a stoppered 10-mL erlenmeyer flask. The temperature on the thermometer will drop at the conclusion of the reaction. Turn the heat off but keep things stirring while the flask cools. Rinse the sides of the condensers with small portions of saturated sodium chloride solution. Collect the rinses in the 10-mL erlenmeyer containing the product mixture. Estimate the volume of the cyclohexene (top) layer. To conserve the product during workup, as a class we are going to collect all of our product mixtures into a single separatory funnel. Allow the layers to settle and remove the aqueous layer through the valve from the bottom. Use a pipet to transfer the cyclohexene layer out the top of the sep funnel to a 25-mL erlenmeyer. Add a couple spatula tips of anhydrous sodium sulfate. Swirl the mixture and wait 5 minutes. Use a pipet to transfer the cyclohexene to another erlenmeyer. Again add a few spatula tips of anhydrous sodium sulfate. Swirl and let settle. If the sodium sulfate isn't clumped and flows freely, simply collect the cyclohexene product in a capped vial. If, however, the sodium sulfate is clumped, repeat the transfer & sodium sulfate drying steps until the sodium sulfate does not clump. We will use this cyclohexene for the electrophilic addition reactions performed by next year's class.
Scoring
Lab Notebook (20 points)
- Mass of cyclohexanol (sig figs)
- Estimate of volume of product
Brief Report (20 points)
- Class perent yield
- IR spectrum of class product overlaiid on NIST
- Conventional mechanism for the reaction with the elementary steps labeled.




