This is "Addition of HBr to Cyclohexene", from the book 32 Weeks of OChem (v. 1.0).
Addition of HBr to Cyclohexene
Learning Objectives
- To know and write the mechanism for electrophilic addition to an alkene.
- To be able to use a water-cooled condenser.
- To perform a microscale reaction at reflux.
- To be able to efficiently use a drying agent.
- To perform an FTIR and use the spectrum as characterization of a product.
Figure 4.1 (Lab) Addition of HBr to Cyclohexene
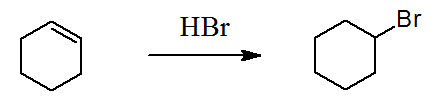
This week's acitivity starts our unit on electrophilic addition reactions. The accepted mechanism begins with the electrophilic addition step forming a carbocation. The electrophile being a proton. This step is followed by a coordination step between the carbocation and a bromide ion nucleophile.
Do all of this procedure IN THE HOOD. Using an Eppendorf pipette, add 0.75 mL cyclohexene (nasty smelling) to a 5-mL conical vial. Clamp the vial in an aluminum heating block such that the vial is centered on a stirring hotplate. Secure to a ring stand. Add a small stir bar. Using a disposable calibrated plastic transfer pipette, add 2.5 mL of HBr. Fit the vial with a water-cooled reflux condenser. Get the mixture stirring briskly and slowly heat to reflux with the water running through the condenser. Start with the hotplate with a setting around 2. If that doesn't reach reflux, you may have to go to a 3 or 4 setting. Heat for 1 hour after attaining reflux. At this time, if you did a good job clamping, you should be able to carefully raise the clamp and lift the entire glassware assembly out of the aluminum heating block and allow it to cool to room temperature. Once cooled, flush down the sides of the condenser and vial with a small amount of diethyl ether from a disposable Pasteur pipet. Be careful of the ether fumes (volatile, flammable and narcotic). Mix the layers with a pipet. Remove the ether layer. Wash the ether layer with saturated sodium bicarbonate solution. Move the ether layer to a 10-mL Erlenmeyer flask, Dry the ether layer with portions of anhydrous sodium sulfate until the salt does not clump and flows freely. Transfer the ether layer to an evaporation dish. Slide it towards the back of the hood for a few minutes. Once the ether has evaporated, obtain an FTIR of your product. If the instructor approves the spectrum, transfer the remainder of your sample to the tared bromocyclohexane collection flask.
Scoring
Lab Notebook (30 points)
- Volumes of reagents (sig figs)
- Mass of product after characterization
Brief Report (30 points)
- Percent yield
- IR spectrum of class product overlaiid on NIST
- Conventional mechanism for the reaction with the elementary steps labeled.




