This is "Acid-Base Extraction", from the book 32 Weeks of OChem (v. 1.0).
Acid-Base Extraction
Learning Objectives
- To become familiar with the operation of a separatory funnel.
- To be able to utilize protonation state as a switch in an extraction.
- To be able to measure melting points.
- To be able to use melting point data to assess purity.
- To write mechanisms for proton transfer steps.
Figure 2.1 (Lab) Digging a Hole

Image Credit: By Mary Attribution-NonCommercial-NoDerivs 2.0 Generic (CC BY-NC-ND 2.0), via Flickr
You can learn a lot digging a hole and refilling it. This week's exercise is pretty much one of those activities. We're going to start with three separate compounds perfectly happy in their own respective bottles. We will mix them together and then spend the rest of the afternoon trying to get them back in their three respective bottles. Along the way you will be learning how to use a separatory (sep) funnel, some quirks of working with diethyl ether, recrystallization techniques, manipulating and measuring the acidity of a mixture and learning how to take melting points. In terms of chemistry, we won't be synthesizing anything new. We'll be working hard trying to get back where we started from in a three hour lab period, but we will be learning a lot of technique.
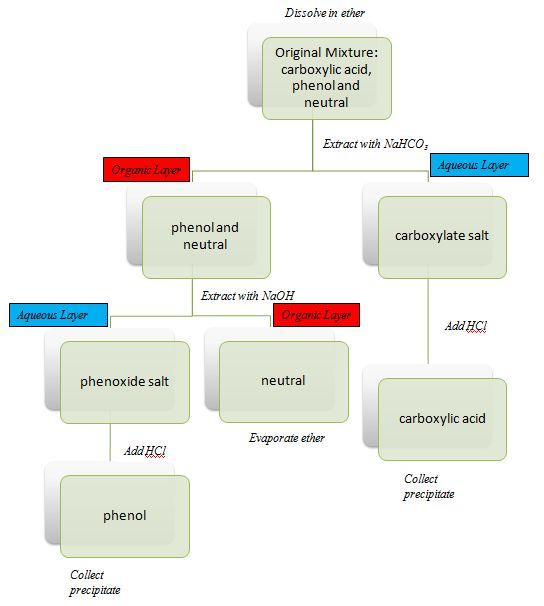
Our separation tool of choice for this exercise will be what is called a separatory (sep) funnel. A sep funnel works by providing two immiscible liquids a chance to separate into layers. A valve is located at the bottom of the flask. As the flask is emptied, the valve is closed upon removing the more dense solvent. One solvent is less polar than the other. In this activity, the less polar solvent will be ether. The more polar solvent will be water. Note that all three solutes in figure 2.3 (Lab) are soluble in ether. Our strategy with an acid base extraction is to nudge one solute into the water by making it charged. We make the solute charged with a simple proton transfer step. Then once we have the charged species separated into the water, we reverse the proton transfer step.
Figure 2.2 (Lab) Separatory Funnel
By 夏侯韬 (Own work) [Public domain], via Wikimedia Commons
Figure 2.3 (Lab) Structures of Compounds
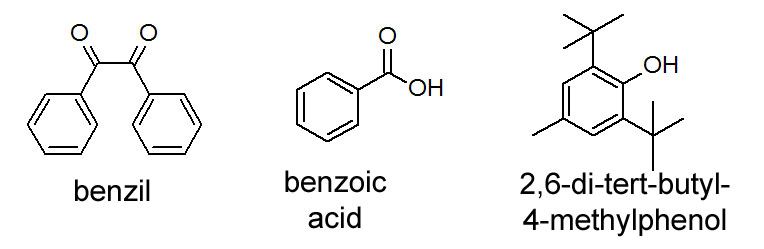
Figure 2.4 (Lab) Separation Schematic
Weigh out and record the amount of about 0.5 g of each of the compounds in Figure 2.3 into a single 100-mL beaker.
Add approximately 15 mL of diethyl ether to the beaker. This is more complicated than it sounds because diethyl ether is harder to work with than water. So first pour the ether from the bottle into another beaker with suitable gradations. Not that it is a trifling task to pour ether from a bottle. Ether has such a high vapor pressure, it is pretty much impossible to use a pipet to transfer diethyl ether. The amount is not terribly important, which is all the more reason the lines on the beaker are satisfactory, but be sure you use enough ether so that all solids gets dissolved in the ether. Not only is the diethyl ether volatile, it is also highly flammable. To make things even more interesting, diethyl ether causes narcosis, and speaking from personal experience, can cause one to collapse with but a single whiff. Throughout this entire experiment, be sure the ether stays in an operating fume hood. There is never a reason for the diethyl ether to be out of the hood. Add about 5 mL of saturated (~1.0 M) NaHCO3 to the beaker and mix the contents well by swirling.
Larger sep funnels fit well in an iron ring fitted on a ring stand. Our smaller sep funnels, however, need to be fitted into an appropriately adjusted clamp attached to a ring stand. The sep funnel needs to fit securely and stand vertically. Pour the contents of the beaker containing the dissolved mixture into a 30-mL sep funnel. As you are familiarizing yourself with the operation of a sep funnel, be sure to wear gloves. Sep funnels can be messy, even in the hands of an experienced user. With one hand holding the cap secure in the top, invert the sep funnel. Keep the nozzle end of the sep funnel pointed to the back of the hood and with your other hand open the valve to vent any gas. Be careful to keep the funnel pointed at the back of the hood as it can spray when you open the valve. Repeat this invert/vent step three more times. Allow the contents to settle into layers. Collect the bottom (aqueous) layer in a 150-mL beaker labeled "A". Repeat the extraction twice more, each with 5-mL portions of 1.0 M NaHCO3 adding the aqueous layers to beaker "A". Repeat the extraction once more but this time use a 5-mL portion of water. Again, add the aqueous layer to beaker "A". Set beaker "A" aside.
Extract the organic layer remaining in the sep funnel with three 5-mL portions of 1.5 M NaOH and one time with water. Collect these aqueous layers in a 150-mL beaker labeled "B". Set beaker "B" aside.
Right now you might think you have just one component of the original mixture left in the ether. This isn't quite the case since water and diethyl ether will mix to some extent. We can get rid of some of the water by one final extraction with saturated NaCl. This water layer can be discarded. To avoid contaminating the ether layer with the water below the valve, transfer the organic layer through the TOP of the sep funnel and into a 150-mL beaker labeled "C". Add anhydrous Na2SO4 to beaker "C" a small spatula tip at a time with swirling until it doesn't clump. Decant the organic layer to an evaporating dish labeled "C". Push the dish to the back of the hood to evaporate the ether.
Dropwise add HCl(conc.) to the contents of beakers "A" and "B" until the pH is 2 or lower. At this point you will likely see significant precipitation which you can collect via vacuum filtration with a Buchner funnel. If no precipitate forms you can put the mixture on ice for a few minutes. If there's still no precipitate, you will need to evaporate some of the water by placing the mixture in an evaporating dish in the fume hood.
Weigh the three separated components.
Obtain the melting point of samples "A", "B" and "C". Also obtain the melting points of the original pure substances. Remember, the term melting "point" is somewhat deceptive. It's really a melting point range that you measure, from the time the contents of the tube collapse until the last crystal is melted.
Scoring
Lab Notebook (20 points)
- Mass of each component of the original mixture (sig figs)
- Melting points of the original substances
- Melting points of the separated substances
- Mass of each component after separation.
- Flowchart of separation (Figure 2.4 (Lab)) with the NAMES of the compounds rather than the families of the compounds.
Brief Report (20 points)
- Table listing percent recovery.
- Statement relating melting point to the purity of each separated substance.
- Conventional mechanisms for the four proton transfer steps you performed.




