This is "Dehydration of 2-Methylcyclohexanol", from the book 32 Weeks of OChem (v. 1.0).
Dehydration of 2-Methylcyclohexanol
Learning Objectives
- To know and write the mechanism for acid catalyzed E2 dehydration of an alcohol.
- To be able to use a Hickman condenser.
- To be able to efficiently use a drying agent.
- To perform an FTIR and use the spectrum as characterization of a product.
- To perform GC analysis of distillation fractions to assess the purity of the product.
Figure 11.1 (Lab) Dehydration of 2-Methylcyclohexanol
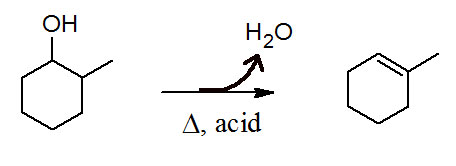
In our last experiment we dehydrated cyclohexanol and suggested an E1 mechanism was the most plausible. In this experiment we will dehydrate 2-methylcyclohexanol. This time we will see an E2 reaction mechanism dominate. In this E2 mechanism the first step is protonation of the alcohol yielding an oxonium ion. The next step is the E2 step yielding the alkene.
Weigh about 4 grams of 2-methylcyclohexanol into a 10-mL round bottom flask. The rest of the procedure takes place in the hood. Alkene products are notoriously stinky and the concentrated phosphoric acid is dangerously corrosive to human tissue. Wear appropriate PPE. Nest the RB flask into an aluminum heating block on a stirring hotplate. Add a stir bar and begin slowly stirring the 2-methylcyclohexanol. Hold the RB upright with a clamp and ring stand. Use a calibrated disposable transfer pipet to add 1.0 mL of 85% H3PO4. Loosely pack the bottom of a Hickman condenser with a suitably sized piece of stainless steel Chore Boy. Attach the Hickman condenser to the RB flask and secure the clamp. Attach the air reflux condenser above the Hickman. Suspend a thermometer such that the bulb hangs just above the Chore Boy. Start heating with the hotplate set around 3-4 to achieve a reflux. Include a thermometer in the aluminum block. Get the temperature of the block up to around 150 °C. Monitor the temperature of the distillate and collect distillate from the Hickman bowl into a 5-mL capped conical vial. Never allow the temperature of the distillate to exceed 95°C. And for safety's sake, never allow a distillation pot to run dry. You should collect around 3.2 mL of distillate. Add a few crystals of solid NaCl to the conical vial until the NaCl no longer dissolves. Add saturated NaHCO3 to neutralize the distillate. Check the acidity by using a glass stir rod to dab distillate onto pH paper. Use a disposable glass Pasteur pipet to remove the top organic layer into a clean, dry 10-mL Erlenmeyer. Add a couple spatula tips of anhydrous sodium sulfate. Swirl the mixture. Continue adding anhydrous sodium sulfate until the sodium sulfate flows freely. Transfer your product to a tared, dry vial and cap securely.
Characterize your product with an FTIR. Also run a GC analysis on your product. .
Scoring
Lab Notebook (20 points)
- Mass of 2-methylcyclohexanol (sig figs)
- Mass of product
Brief Report (20 points)
- Percent yield
- IR spectrum of product overlaid on NIST
- GC chromatogram
- Conventional mechanism for the reaction with the elementary steps labeled.




