This is "Synthesis of o-Formylphenoxyacetic Acid", from the book 32 Weeks of OChem (v. 1.0).
Synthesis of o-Formylphenoxyacetic Acid
Learning Objectives
- To know and write the SN2 mechanism for formation of an ether (Williamson Ether Synthesis)..
- To perform a simple distillation using a Hickmann condenser.
- To perform a microscale reaction at reflux.
- To be able to efficiently use a fritted glass funnel and perform a vacuum filtration to isolate a crystalline product.
- To be able to use a mini-press to make a KBr disk of a solid sample.
- To perform an FTIR and use the spectrum as characterization of a product.
- To characterize a product by melting point. .
Figure 7.1 (Lab) Synthesis of o-Formylphenoxyacetic Acid from Salicaldehyde and 2-Chloroacetic Acid
This week's acitivity is our first SN2 reaction. A proton transfer elementary step deprotonates the phenol (and the alkyl halide) and gives a nucleophile for the bimolecular nucleophilic substitution elementary step. The synthesis is concluded with another proton transfer step giving the product. This last proton transfer step also helps with the isolation of the product since the neutral acid is less soluble in water than the anion. This lab is a doozy but can still be completed in a 3-hour lab period if your ducks are in a row.
Precisely 0.48 g of 2-chloroacetic acid has been preweighed into a 10-mL round bottom flask awaiting you in the hood. This is not only for the sake of time. The 2-chloroacetic acid is nasty stuff. It's one of those substances than can do grievous harm, even causing death, by absorption through your skin. WEAR GLOVES. Do the remainder of this procedure IN THE HOOD. Add a stir bar, 0.50 mL salicylaldehyde, 4 mL of H2O and 0.40 mL 50% NaOH to the rb flask. Fit the flask with an air reflux condenser. Secure the flask to a ringstand in an aluminum heating block on a stirring hotplate. A yellow solid forms but it will dissolve with heating. While stirring, bring the temperature of the block up to 110 °C. Heat for one hour AFTER achieving a boil. Allow the apparatus to cool sufficiently so you can safely remove the reflux condenser. Add 0.95 mL concentrated HCl. Fit a Hickmann condenser and air reflux condenser and a thermometer and restart the heating. A milky substance containing a mixture of water and unreacted salicylaldehyde will collect in the bowl of the Hickmann condenser. Keep an eye on the temperature and after a mL or two of distillate has been collected the temperature will drop. At this point it's time to cool things down. Once you can handle the rb flask, carefully clamp it in an ice water bath. Use a stir bar retriever to remove the stir bar. After about 20 minutes in the ice water, you should have crystals. Collect the crystals using a fritted glass funnel and vacuum. Allow the crystals to dry on the vacuum. Be sure to use a clean flask to collect your filtrate as crystals can sometimes form after passing through the funnel. Weigh your product. Measure the melting point of your product and obtain an IR spectrum from a KBr disk.
Results
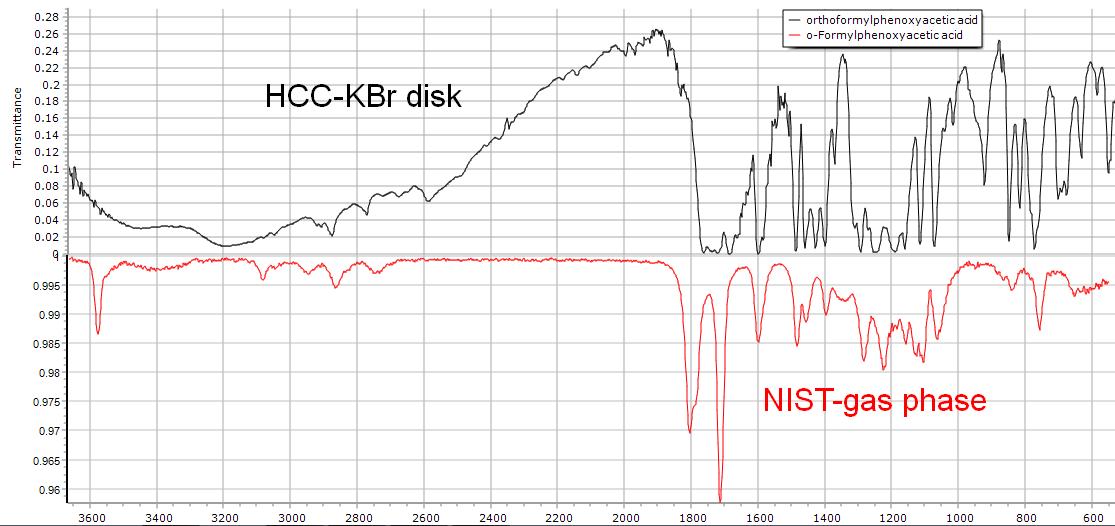
The product is crude at this point and there isn't time for a recrystallization. Although literature values for the melting point are around 130-135 °C, our crude products typically begin melting around 120°C. NIST does not offer a condensed phase IR spectrum of our product. Use the spectrum below obtained on our FTIR for comparison.
Figure 7.1 (Lab) FTIR Spectrum of Crude Product and NIST Comparison
Scoring
Lab Notebook (40 points)
- Weight and volumes of reagents (sig figs)
- Distillation temperature
- Mass of product before characterization (sig figs)
- Percent yield
- IR spectrum of product overlaid on NIST-obtained spectrum.
- Melting Point RANGE




