This is "Spectroscopic Detection of Nitrogen in Organic Compounds" from the book 32 Weeks of OChem (v. 1.0).
Spectroscopic Detection of Nitrogen in Organic Compounds
or
"Finding N..."

By zannaland (Disney's Art of Animation Resort) [CC BY-SA 2.0 (http://creativecommons.org/licenses/by-sa/2.0)], via Wikimedia Commons
Learning Objectives
- To interpret a mass spectrum of a compound which may contain nitrogen.
- To know characteristic IR bands for amines, amides and nitriles.
- To be able to associate 13C and 1H NMR chemical shift information with the presence of nitrogen atoms in a compound.
Way back in general chemistry we learned the valence of nitrogen is three. Meaning a nitrogen atom forms three bonds in neutral compounds.
Table 4.1 (Spec) Organic Families with Nitrogen Atoms
MS
The trivalent nature of of nitrogen gives us a way to spot nitrogen atoms in the mass spectrum of molecules having an odd number of nitrogen atoms. The mass of the M+ for compounds having an odd number of nitrogens will be odd. Further, many of the principle fragments in the MS will have even masses. This is exactly opposite of what we find in compounds having no nitrogens. Compounds without nitrogens have an even M+ mass and odd mass principle fragments. Another consequence of the trivalent nature of nitrogen is the sign of its term in the rings + double bond formula. Apply the r+db formula to the example compounds in Table 4.1(Spec).
IR (FTIR)
N-H stretches
Recall from the Spectroscopic Detection of Oxygen in Organic Compounds section how recognizable the presence of an OH (hydroxyl) group is in an infrared spectrum (Santa's belly). An N-H stretching band gives a similar, though distinctive, appearance. Looking up the bond strengths in Table 2.1 (Spec) we find the N-H bond to be 391 kJ/mol. This is much weaker than the O-H (467 kJ/mol) and even the C-H (413 kJ/mol). Judging from the bond strengths alone we would expect the N-H stretching band to be buried in the low end wavenumbers of the C-H stretching bands. However, due to hydrogen bonding in the condensed phases, the N-H stretch is in about the same region of the spectrum as the O-H stretch. Be aware, just because a compound has a nitrogen atom does not mean it will have an N-H stretch. Amines and amides are classified as primary, secondary, or tertiary. The designation refers to the number of alkyl groups bonded to the nitrogen atom. In primary amines and amides, the nitrogen is bonded to two hydrogen atoms and one alkyl (or acyl in the case of an amide) group; in secondary amines and amides, the nitrogen is bonded to one hydrogen and two alkyl (acyl) groups; and in tertiary amines and amides, the nitrogen is bonded to three alkyl (acyl) groups. So, the only compounds from table 4.1 (Spec) that give N-H stretches are the primary and secondary amines and amides. Whereas the C-H stretch is pretty typically 2800-3000 cm-1, because of hydrogen bonding, the N-H stretch(es) is (are) a little higher and between 3500 and 3200 cm-1. Whereas the C-H stretch gives a relatively sharp absorption, the N-H stretch is broadened in condensed phases due to hydrogen bonds. Also, N-H stretching bands tend to be weaker in intensity. Primary amines typically give 2 N-H stretching bands, secondary amines give only one. Primary amides give 2 N-H stretching bands. Secondary amides are more variable with respect to the number of bands due to dimers and polymers possible in some configurations. It is easy to distinguish an amine from an amide by looking for the C=O stretch around 1650 cm-1.
Let's compare spectra for some isomeric amines with the molecular formula C4H11N.
Figure 4.1 (Spec) Infrared Spectra of Amines
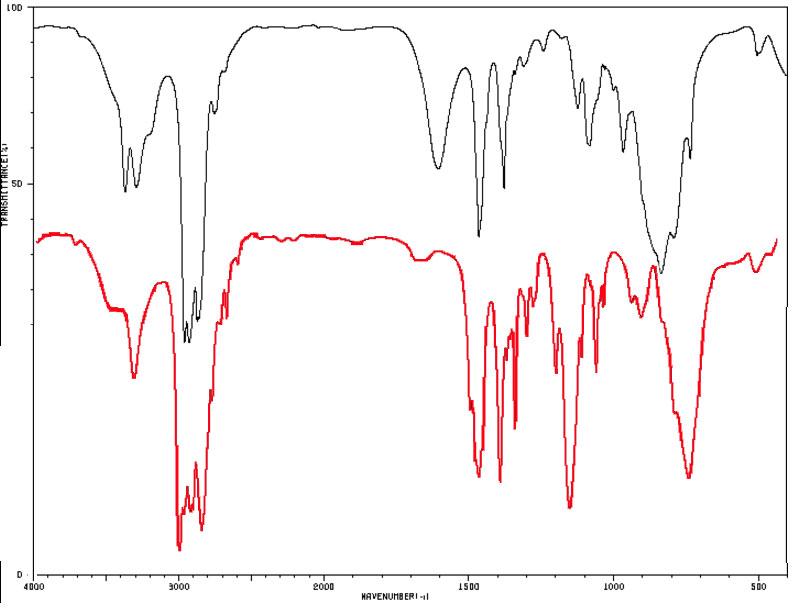
The black line spectrum (upper most) is of 1-butanamine (a primary amine) and the red line (lower most) is the spectrum for diethyl amine (a secondary amine). By SDBSWeb : http://sdbs.db.aist.go.jp (National Institute of Advanced Industrial Science and Technology, accessed August 13, 2017
And now let's look at the IR spectra of some isomeric amides with the molecular formula C4H9NO.
Figure 4.2 (Spec) Infrared Spectra of Amides
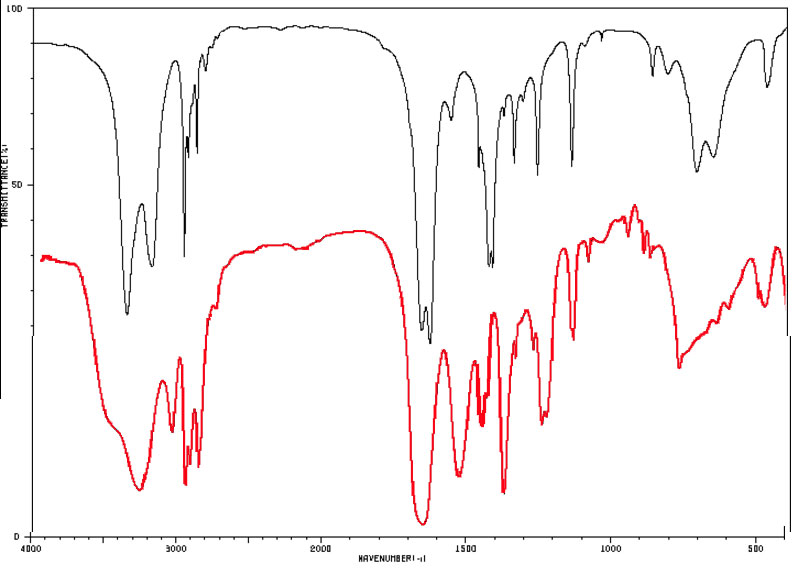
The black line spectrum (upper most) is of butanamide (a primary amide) and the red line (lower most) is the spectrum for N-propyl formamide (a secondary amide). By SDBSWeb : http://sdbs.db.aist.go.jp (National Institute of Advanced Industrial Science and Technology, accessed August 14, 2017
C-N Stretches, N-H bends & Various Combinations Thereof
Medium intensity C-N stretches, exactly analogous to the C-O stretches we saw last time, are seen in primary and secondary amines in the 1250-1000 cm-1 region. N-H bending (scissoring) in primary amines gives a medium intensity band around 1610 cm-1. Practically no N-H scissoring is observed in secondary amines. N-H wagging is seen in primary and secondary amines with a broad, medium intensity band between 900 and 600 cm-1. For the amides, the primary amides give a NH2 bending doublet around 1600 cm-1. Secondary amides give the N-H bending band around 1550 cm-1.
C≡N Stretches
Nitriles, by virtue of their C≡N group, give a distinctive band of medium intensity in the region of 2240-2220 cm-1.
Figure 4.3 (Spec) Infrared Spectrum of a Nitrile
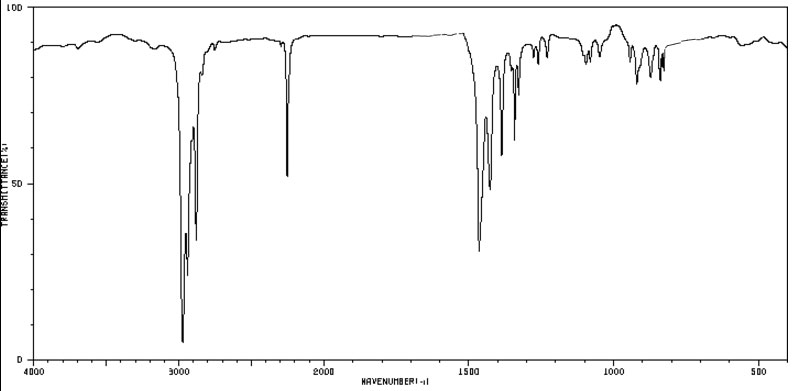
The spectrum is of butanenitrile. By SDBSWeb : http://sdbs.db.aist.go.jp (National Institute of AdvancedIndustrial Science and Technology, accessed August 14, 2017
NMR
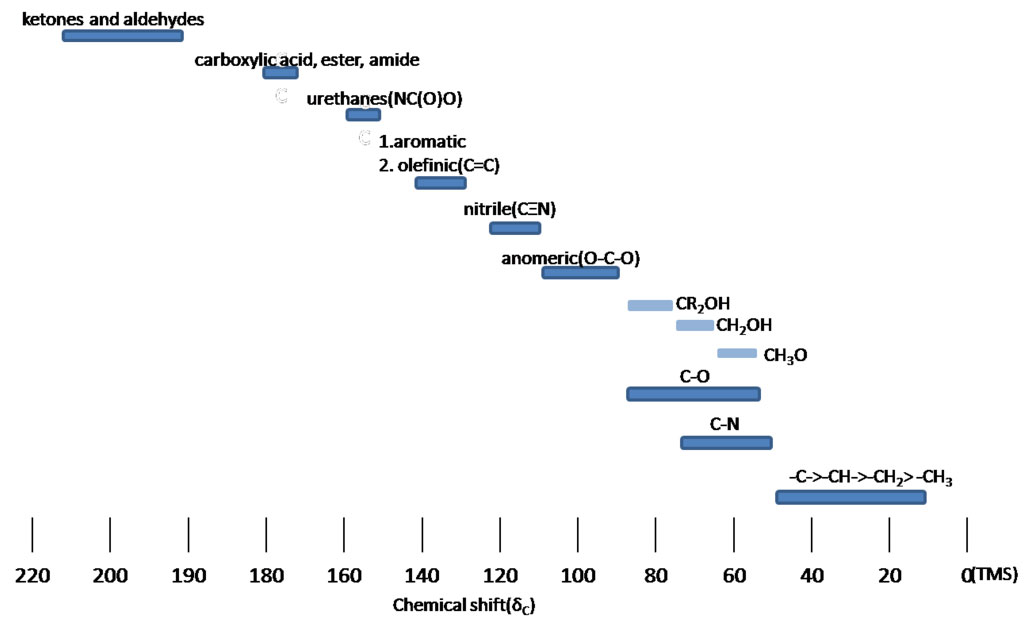
The 1H NMR is not terribly helpful when it comes to spotting the presence of nitrogen. Yes, the proton will give a signal. It is usually rather broad and the chemical shift is quite variable over practically the entire spectrum. One doesn't look at a 1H NMR spectrum and have the nitrogen's proton just hop out at you. Instead, it's more often that after the structure has been suggested as a possibility, one looks and sees an unexplained signal and attributes that to the proton on the nitrogen. 13C NMR however can give a much clearer indication of the presence of a carbon bonded to a nitrogen.
Figure 4.4 (Spec) 13C NMR Chemical Shifts
Example 4.1 (spec)
Identify the unknown compound using the following four spectra.
Figure 4.5 (Spec) Mass Spectrum
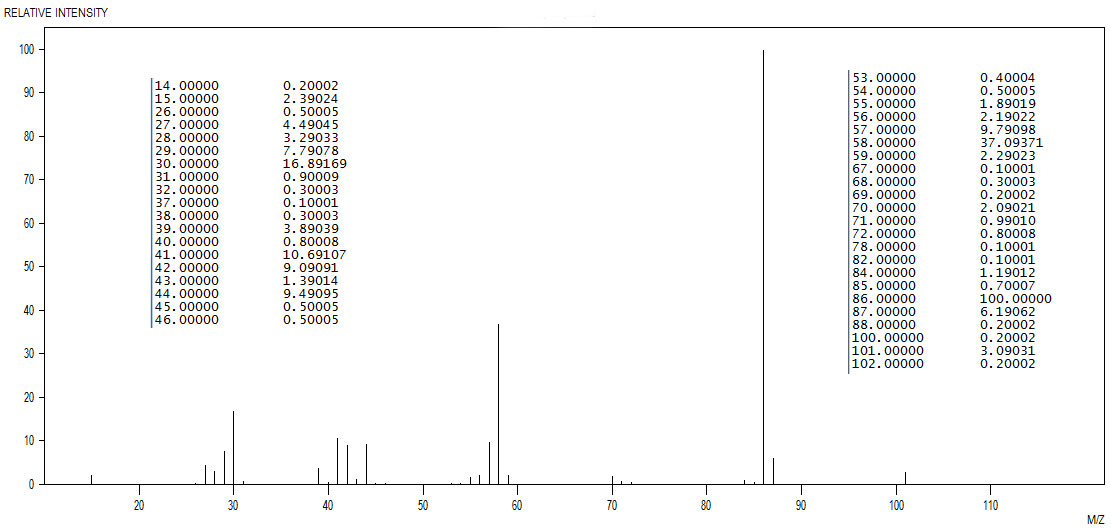
Figure 4.6 (Spec) IR Spectrum
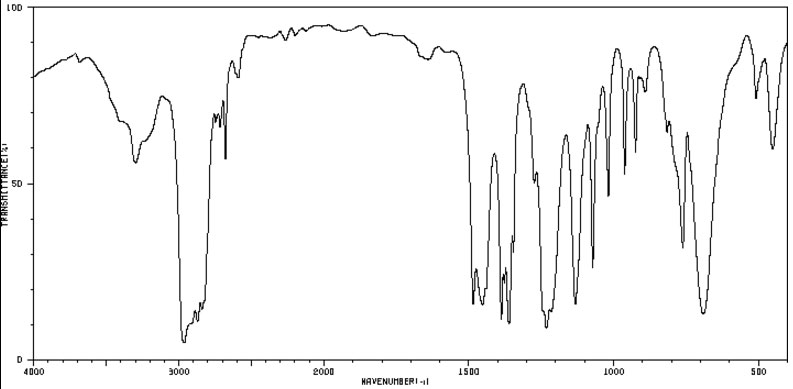
By SDBSWeb : http://sdbs.db.aist.go.jp (National Institute of AdvancedIndustrial Science and Technology), accessed August 15, 2017
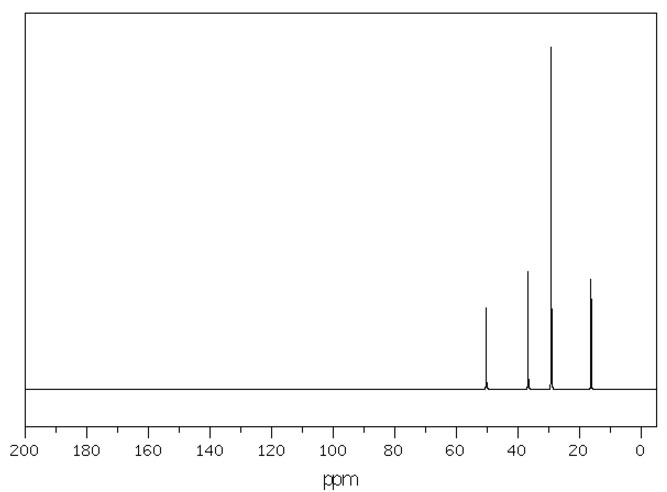
Figure 4.7 (Spec) 13C NMR Spectrum
By SDBSWeb : http://sdbs.db.aist.go.jp (National Institute ofAdvancedIndustrial Science and Technology), accessed August 15, 2017
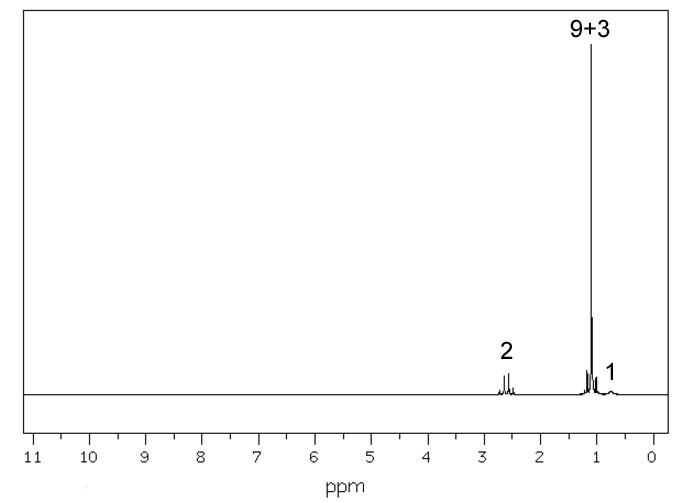
Figure 4.8 (Spec) 1H NMR Spectrum
By SDBSWeb : http://sdbs.db.aist.go.jp (National Institute ofAdvancedIndustrial Science and Technology), accessed August 15, 2017
Answer
Step 1: Identify the probable M+ ion in the mass spectrum (Figure 4.5 (Spec)). The most likely candidate is at 101 m/z. The 13C isotopic peak looks like it is buried in the noise so we are unable to use it to calculate the number of carbon atoms. We are however reassured that 101 is the M+ by the presence of the base peak at M-15, which is 86 m/z. ding 100 by the intensity at 88. The fact that 101 is an odd number and we have major fragments at 86, 70 and 58 m/z leads us to suspetc the presence of an odd number of nitrogen atoms.
Step 2: The MS gives us a pretty good idea the molecular weight of our unknown is 101; however, it does not allow us to calculate a molecular formula. So for this unknown, we ought to take a look at the 1H NMR (FIgure 4.8 (Spec)) integration and see how many protons might be in our compound. It looks like 15 protons. With 15 amu from the protons and 14 amu from the nitrogen, we'd need 72 amu to get to 101. That would mean 6 carbons and a probable molecular formula would be C6H15N. What about an oxygen or two? One oxygen would leave 56 amu for carbon and that's not a multiple of 12. Two oxygens would leave 48 amu for carbon. That would work. So another likely molecular formula would be C4H15NO2.
Step 3: Applying the rings + double bonds formula to either C6H15N or C4H15NO2 gives 0 rings or double bonds.
Step 4: Checking the IR (Figure 4.6 (Spec)), the band at 3220 cm-1 makes us think the N-H stretch of a secondary amine. This is confirmed by the C-N stretch at 1220 cm-1, the lack of a significant N-H scissoring at 1610 cm-1 and the broad band around 690 cm-1 associated with N-H wagging. Further, the band at 1520 cm-1 indicates a secondary amine and not a primary amine since it is a single sharp band at a lower wavenumber than one would expect for a primary amine (and it's not a doublet). Nothing in the IR indicates the presence of oxygen. There's no Santa's belly so it's definitely not an alcohol. There could be some sneaky ether functionality. C-O stretches and C-N stretches look similar.
Step 5: We still need to nail down our molecular formula. Let's consider the 1H NMR (Figure 4.8 (Spec)). If there is an ether functionality we'd see a peak around 3 or 4 ppm. The closest thing is at 2.6, but that is more attributable to an H on a carbon attached to a nitrogen. It's now safe to say there's no oxygen. Our molecular formula is definitely C6H15N.
Step 6: Consider the 13C NMR (Figure 4.7 (Spec)). We see 4 distinct peaks. The signal at 51 ppm is clearly from a carbon connected to a nitrogen. We know it's a secondary amine, so the peak at 36 must also be from a carbon attached to a nitrogen. The peak at 29 and the peak at 16 must be from carbons distant. The fact that there are 4 distinct peaks indicates some symmetry in the molecule. The peak at 3.7 ppm is maybe a little high for an ether, but still within the range indicated in Figure 3.3 (Spec).
Step 7: Pause and consider the possibilities by drawing structural isomers of C6H15N that are secondary amines and possess symmetry that presents just four distinct carbons.
Figure 4.9 (Spec) Structures of Candidates
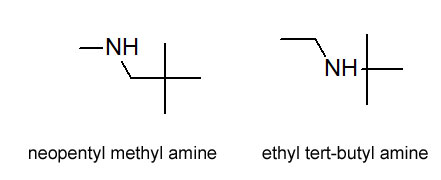
Reconsidering the 1H-NMR with an eye again at the integration. The interation of 1 at around 0.8 ppm must be from the hydrogen on the nitrogen. The 9 + 3 integration at 1.1 ppm could be the H's of a tert-butyl group and the integration of 2 from the protons on a methylene (-CH2-) group. Unfortunately, this integration is completely consistent with both choices. The presenece of the tert-butyl group is confirmed in the IR spectrum (Figure 4.6 (Spec)) by the doublet in the 1350-1385 cm-1 region.
And it appears we are stuck. There are a couple of good observations we could make that would let us distinguish these two. We could look at the fragmentation pattern in the MS. We could also look at the peak splitting patterns in the 1H NMR. Unfortunately, these will have to wait until a little later in the semester. So, at this point in our learning, the best we can do is guess. Or, we could look them up in the SDBS, but that's cheating!




