This is "Addition of HBr to Cyclohexene", from the book 32 Weeks of OChem (v. 1.0).
Synthesis of tert-Pentyl Chloride
Learning Objectives
- To know and write the SN1 mechanism for halogenation of an alcohol.
- To perform a simple distillation using a Hickmann condenser.
- To perform a microscale reaction at reflux.
- To be able to efficiently use a drying agent.
- To perform an FTIR and use the spectrum as characterization of a product.
- To perform a GC analysis of the purity of the product.
Figure 6.1 (Lab) Synthesis of t-Pentyl Chloride from t-Pentyl Alcohol
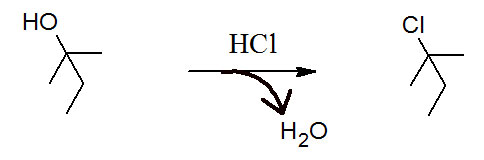
This week's acitivity is our first SN1 reaction. This mechanism starts with a proton transfer elementary step which is followed by a rate-limiting heterolysis elementary step forming a carbocation. The last step is to attack the electrophilic carbocation with a chloride nucleophile in a coordination elementary step.
Weigh approximately 4 mL of tert-pentyl alcohol into a 50-mL Erlenmeyer flask. Do the remainer of this procedure IN THE HOOD. Add 10 mL conc. HCl *caution* to the Erlenmeyer. Add a stir bar, stopper the flask with a cork, and stir at room temperature. After 1 hour, add NaCl(s) until saturated. It only takes a tiny amount of the NaCl. Add a few crystals at a time until they appear not to dissolve. Leaving the undissolved crystals of NaCl behind, transfer the contents of the Erlenmeyer to a 30-mL separatory funnel. Drain the aqueous lower layer (remember it's still essentially conc. HCl) to a waste beaker. Wash the organic layer with water, followed by sat'd NaHCO3, followed by water. Be sure to drain all the water from the separatory funnel on the last washing. Using a pipet from the top of the separatory funnel, remove the organic layer and transfer to a 10-mL Erlenmeyer flask. Add a scoop or two of anhydrous sodium sulfate. Transfer the liquid to another 10-mL Erlenmeyer flask. Add another scoop of anhydrous sodium sulfate. Continue the transfer and drying steps until the sodium sulfate flows freely and does not clump. This could take four or five 10-mL Erlenmeyer flasks. Transfer the dried organic layer to a 3-mL conical vial nested in an aluminum heating block on a hot plate. Add a small stir bar in place of a boiling stone. There's no need to stir. Clamp in a ring stand and add a Hickmann condenser and an air reflux condenser. Suspend a thermometer down the condensers so the bulb is below the Hickmann bowl. Heat the mixture. The product distills at around 80-85 °C. Often in this distillation, unlike most distillations, there is no observed drop in the temperature at the conclusion of the distillation; however, as always, NEVER let the distillation vessel go dry. Remove the contents of the Hickmann condenser's collection bowl. Weigh the amount of product. Obtain an IR spectrum and run a GC of your product.
Scoring
Lab Notebook (40 points)
- Weight of reagents (sig figs)
- Distillation temperature
- Mass of product before characterization (sig figs)
- Amount of sample injected to GC (sig figs)
- Percent yield
- IR spectrum of product overlaid on NIST-obtained spectrum.
- GC chromatogram with calculation of purity.




