This is "Week 1", section 1.3 from the book 32 Weeks of OChem (v. 1.0).
1.3 Reactions
Learning Objectives
- To be able to compare and contrast a reaction equation with a reaction mechanism.
- To be able to use curved arrow formalism for pushing electrons in a reaction mechanism.
- To distinguish nucleophiles and electrophiles.
- To memorize the electron flow for a proton transfer elementary step.
- To use a pKa table to predict favorable and unfavorable proton transfer steps.
Thank heaven for chemical change. If it weren't for chemical change, chemistry would be pretty boring. In fact, if it weren't for chemical change, life could not exist. All life forms are essentially fuel cells. Recall fuel cells can be considered refillable power supplies. Like all animals. we "refill" by consuming the products of photosynthesis from plants. Chemical change can be described in a number of ways. In general chemistry you learned to write balanced chemical equations to describe chemical change. Although balanced equations still have an important spot in your continuing study of chemistry, there are other ways of describing chemical change besides simply listing the reactants and products of a chemical process. In this course, we will dedicate a large amount of study to reaction "mechanisms." Mechanisms are a series of what are called elementary steps. The sequence of steps called the mechanism illustrates the flow of electrons as bonds are broken and made to turn reactants into products. Although there are thousands and thousands of chemical reactions, there are a relatively few number of elementary steps. If we learn these elementary steps and learn how to logically connect the elementary steps, the myriad of reactions presented in organic chemistry is much less intimidating. Here at the beginning of the course, each week we will be tackling one or two of these elementary steps. In this section we will consider our first elementary step which we call "proton transfer."
Nucleophiles, Electrophiles and the Curved Arrow Formalism for a Proton Transfer Step
Many elementary steps have a species identified as a nucleophile. The nucleophile always has a pair of electrons (either unshared or in a pi bond) that can be donated to a species called an electrophile. As a notation, chemists use something called curved arrow formalism to show the flow of electrons. The curved arrow ALWAYS starts from electrons. NEVER start a curved arrow from an atom, only start a curved arrow from either bonding or unshared electrons. In applying curved arrow formalism to the elementary step called proton transfer, the curved arrow must start from an unshared pair of electrons on the base. ALL BASES ARE NUCLEOPHILES. This curved arrow must point to the removable hydrogen atom on the acid. This dissociable proton on the acid is the electrophile. Then another curved arrow must be started from the covalent bond joining the hydrogen to the rest of the acid. This second curved arrow must point at the atom that was formerly bonded to the hydrogen of the acid. The first curved arrow showed a bond forming from an unshared pair. The second curved arrow shows a bond breaking and turning into an unhshared pair on the conjugate base. Note how the red pair of electrons in figure 1.3-1 becomes a red bond and the blue bond in figure 1.3-1 becomes an unshared pair.
Figure 1.3-1 A Proton Transfer Elementary Step
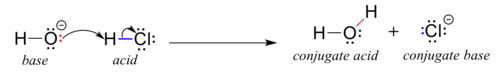
Image Credit: Organic Chemistry with a Biological Emphasis, Soderberg, Creative Commons Attribution-Noncommercial-Share Alike 3.0 United States License
Recall our acid-base definitions from general chemistry. The Brønsted–Lowry defiinition takes the perspective of the proton. The Lewis definition takes the perspective of the electron. Recall that all Brønsted–Lowry acids are also Lewis acids, but not all Lewis acids are Brønsted–Lowry acids. For the proton transfer step we are necessarily limited to the Brønsted–Lowry acids and bases; however, when we draw our curved arrows we are showing the reaction from the perspective of the electron pairs. So our depictions are Lewis theory applied to the Brønsted–Lowry acid-base reactions. ALL ORGANIC MECHANISMS ARE ELECTRONIC as opposed to NUCLEAR. This is why you always start your arrows from electrons, not nuclei. The biggest rookie mistake in writing mechanisms is pointing arrows the wrong way. If you always start making arrows from electrons, you won't make this rookie mistake.
In Figure1.3-1 the base, hydroxide ion, a strong base; likewise, hydrochloric acid is a strong acid. Of course such a reaction will go to completion as written. This seems a silly question, but just ask yourself, how come OH- doesn't give up it's proton and how come H-Cl doesn't accept the proton from OH-? Why don't we get O2- and H2Cl+ as products? Recall, the laws of thermodynamics determine the species that will predominate at equilbrium. To apply the laws of thermodynamics to proton transfer reactions, one uses a table of pKa values.
Table 1.3-1 Representative Acid Constants
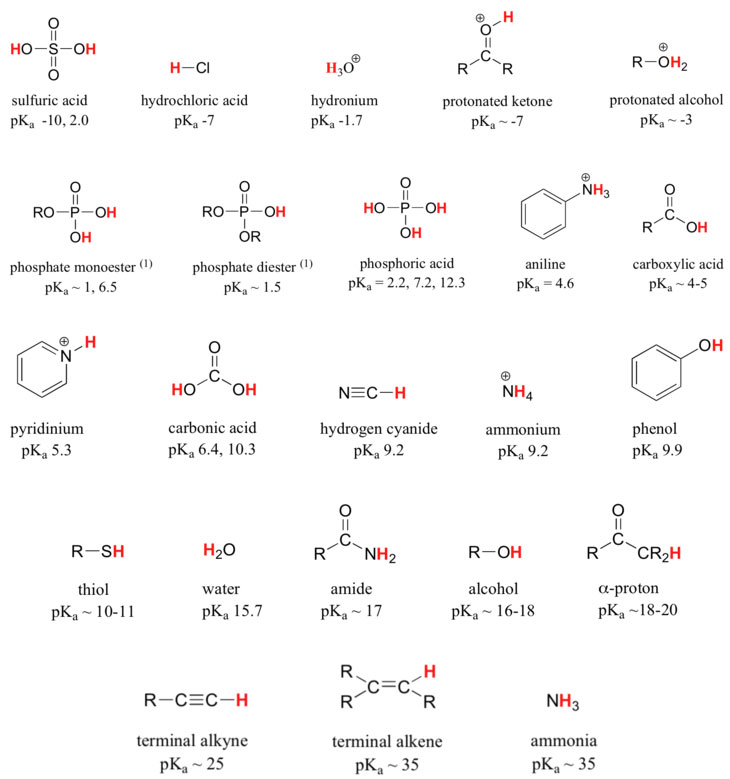
Image Credit: Organic Chemistry with a Biological Emphasis, Soderberg, Creative Commons Attribution-Noncommercial-Share Alike 3.0 United States License
To apply Table 1.3-1 to the reaction in figure 1.3-1, one finds the pKa's for the acid and the conjugate acid of the base. In this case, we compare the pKa of H-Cl (-7) with the pKa of H-OH(15). A pKa of -7 represents an acid 10,000,000,000,000,000,000,000 (1022) times as strong as an acid with a pKa of 15. To estimate a value for the equilibrium constant we can use the relation:
where ΔpKa = pKa of the conjugate acid of the base (product) - pKa of the acid (reactant).
In this case ΔpKa = 15 - (-7). So K = 1022, and this reaction will go to completion. A general rule of thumb is a favorable differential of about 5 pKa units gives a proton transfer reaction that is essentially going to completion. In other words as long as the acid's pKa is 5 units or more lower than the conjugate acid of the base, the reaction will go to completion. A favorable pKa difference of between 0 and 5 means there will be a mixture of reactants and products. But consider reactions that go the wrong way, that is, where the substance acting as an acid has a higher pKa than the conjugate acid of what is to be the base. Although such reactions are definitely unfavorable, they can be important, even up to a difference of as many as 10 pKa units in the wrong direction, where K = 0.0000000001. Thermodynamically unfavorable does not mean impossible or never.
Reactions that have a favorable difference in pKa, where the pKa of the acid is lower than the pKa of the conjugate acid of the base, will always yield a conjugate acid that is a weaker acid than the original acid and a conjugate base that is a weaker base than the original base.




