This is "Week 1", section 1.1 from the book 32 Weeks of OChem (v. 1.0).
1.1 Structure
Learning Objectives
- To recognize functional groups of hydrocarbons and halogenated hydrocarbons.
- To be able to draw organic structural formulas (Complete, condensed and bond-line).
- To be able to assign names to organic structures from memory.
- To recognize structural (consititutional) isomers.
Welcome! The purpose of this supplement is to provide an electronic resource arranged according to the lecture schedule. The first "1" in the "1.1" assigned this section indicates the week in the semester. The "1" following the dot indicates the subsection where all the structure, nomenclature, convention and trivia for the week is placed. Subsection 2 is where we put the "how do we know" material. Subsection 3 is for reaction mechanisms and chemical synthetic schemes. Subsection 4 is for biological applications of organic chemistry. This organization with the same four subsections is followed for all 32 weeks of the two semesters. So, this book has 128 pages, of which some are longer than others. Although this supplement is not intended to be a textbook, it is used as a central location for all the material required for the quiz questions in Moodle. Whereas a textbook has lots of detail arranged with logical transitions, this supplement will at times seem brief, general and fragmented. This supplement is also continuously being revised. You, the reader, are encouraged to give feedback and help steer the evolution of 32 weeks of OChem.
A JSMol panel appears at the top of every "n.1" page in this textbook. Although the models take a long time to load and might hog a little memory on your device, spending some time playing with these little models will take you leaps and bounds towards developing some essential spatial reasoning skills required for understanding organic chemistry. The models are interactive with your mouse. Give them a drag or click or ctrl+click or a right click and explore. Think about the shapes and bond angles that are presented. Be on the lookout for planes of atoms in the models.
Table 1.1-1 Classes of Organic Compounds (MEMORIZE)
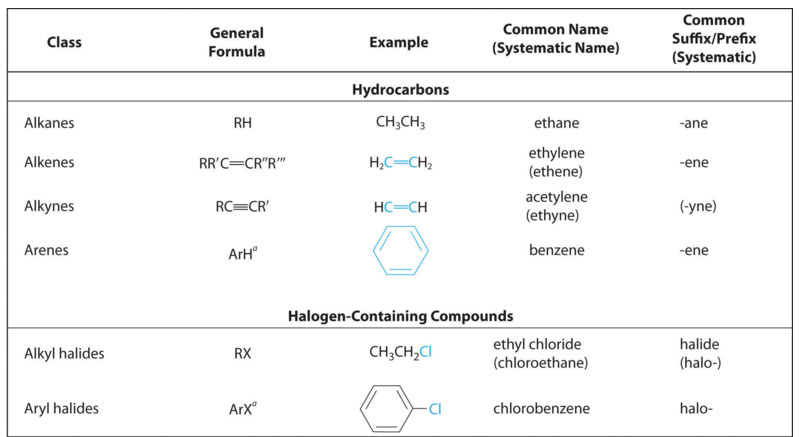
The above classification of organic compounds distinguishes families. Similar to the way elements in the same family of the periodic table exhibit similar chemistry, so to do the compounds of the same organic family. The reason elements of the same family exhibit the same chemistry is due to the arrangement of the valence electrons being the same for all the elements of the group. Likewise, compounds of the same organic family have the same chemistry because they have the same arrangement of the valence electrons. In an element, the valence electrons are positioned around an atomic nucleus. In an organic molecule, the valence electron are distributed within a molecular species. As a an organic family, the alkanes are notoriously unreactive. However, when functional groups are present, the family is considered much more reactive. It is the presence of these functional groups that distinguishes one family of organic compounds from another. The alkanes have no functional group. Alkenes have a C=C, alkynes have a C≡C, arenes have a benzene ring, and alkyl halides have an -X, where X indicates a halogen atom. The "R" in table 1.1-1 designates an alkyl group, shown in table 1.1-5.
Organic chemists use a systematic nomenclature to indicate what atoms are present in a compound and how those atoms are connected in the molecule. Organic chemists can also indicate the atoms and how those atoms are connected with structural formulas. So, yes, there is redundancy in having both names and structures; however, imagine trying to describe a structure to another person over the phone, or while passing one another in the hallway, or in a text message. Also, if we only had structures, how would we search databases or order reagents? We can't always draw structures, sometimes we also need names.
The Parts
IUPAC names have different parts categorized as ending or prefix.
Ending: The ending of the name indicates the functional group present in the molecule. Since alkanes have only carbons and hydrogens connected by single bonds, there is no functional group to indicate and we end the name with -ane.
Prefix(es): Any number of prefixes can be applied to the ending of an alkane.
- The prefix immediately preceding the ending indicates the number of carbons in the parent chain.
- The prefix "cyclo" indicates a ring structure.
- Prefixes, usually accompanied by numbers, are used to indicate branches in the carbon chain.
- Prefixes, again usually accompanied by numbers, are used to indicate halogen atoms that have been substituted for hydrogens.
Note the Pattern
Every organic compound has a unique systematic name and a structure.
Prefix for the Number of Carbons in the Parent Chain
The parent chain is the longest continuous chain of carbon atoms. Below are the names of the first twenty alkanes with their bond-line structures. Note the suffix is -ane in all cases. The prefix indicates the number of carbons.
Table 1.1-2 The First Twenty Straight-Chain Alkanes (MEMORIZE)
| Name | Molecular Formula | Condensed Structural Formula |
|---|---|---|
| Methane | CH4 | CH4 |
| Ethane | C2H6 | CH3CH3 |
| Propane | C3H8 | CH3CH2CH3 |
| Butane | C4H10 | CH3(CH2)2CH3 |
| Pentane | C5H12 | CH3(CH2)3CH3 |
| Hexane | C6H14 | CH3(CH2)4CH3 |
| Heptane | C7H16 | CH3(CH2)5CH3 |
| Octane | C8H18 | CH3(CH2)6CH3 |
| Nonane | C9H20 | CH3(CH2)7CH3 |
| Decane | C10H22 | CH3(CH2)8CH3 |
| Undecane | C11H24 | CH3(CH2)9CH3 |
| Dodecane | C12H26 | CH3(CH2)10CH3 |
| Tridecane | C13H28 | CH3(CH2)11CH3 |
| Tetradecane | C14H30 | CH3(CH2)12CH3 |
| Pentadecane | C15H32 | CH3(CH2)13CH3 |
| Hexadecane | C16H34 | CH3(CH2)14CH3 |
| Heptadecane | C17H36 | CH3(CH2)15CH3 |
| Octadecane | C18H38 | CH3(CH2)16CH3 |
| Nonadecane | C19H40 | CH3(CH2)17CH3 |
| Eicosane | C20H42 | CH3(CH2)18CH3 |
Table is from http://chemwiki.ucdavis.edu/Organic_Chemistry/Hydrocarbons/Alkanes/Nomenclature_of_Alkanes published under a Creative Commons 3.0 License.
All these letters and subscripted numbers make for some fairly complicated structures, even when we use the condensed structures shown in table 1.1-2. Alternatively, and almost always more elegantly, chemists use what are called bond-line structures or sometimes skeletal structures. These structures show only the carbon-carbon bonds represented as lines. For hydrocarbons, everything else in the structure is implied and understood. When heteroatoms are in the structure, all bonds on the heteroatom and any hydrogens attached to the heteroatom are explicitly shown. Some bond-line structures are shown in table 1.1-3 and 1.1-4 below.
Table 1.1-3 Bond-line (skeletal) structures
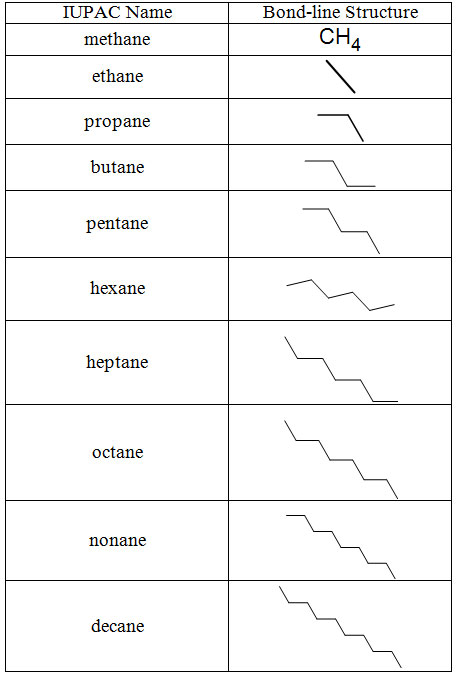
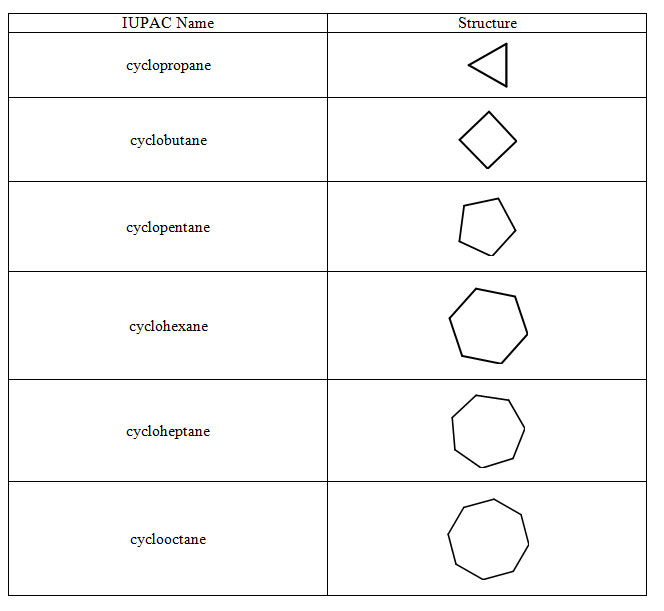
Table 1.1-4 Cycloalkanes
Prefixes for Branches in the Parent Chain
When a carbon is attached to more than two other carbons, we call this a branch point and this compound is a branched alkane. This means there are more carbons than just those in the parent chain. Below is a table of some alkyl groups which can be used as branches on parent chains. For the purpose of the example, the groups are attached to a cyclpentane ring. In fact, these groups can be attached to any carbon of any alkane.
Table 1.1-5 Alkyl Groups on a Cyclopentane Parent Chain

Branches are not limited to cyclic structures. Branches are also found on acyclic (not ring) structures. When the branches are on acyclic structures, one needs to take care when identifying the parent chain. Also, a number is required to indicate where the branch is in the structure consider the following examples.
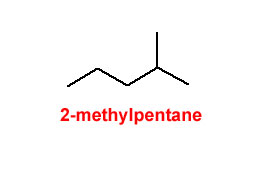
Figure 1.1-1 Methyl Groups on a 5-Carbon Chain
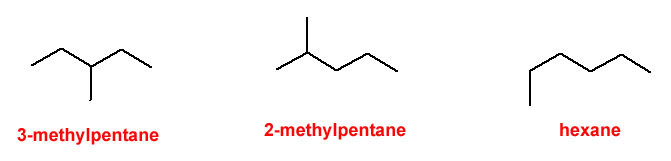
Beware of the rookie mistake often made when naming the rightmost structure. There is a tendency to try and name that rightmost structure as 1-methylpentane; however, the position of the methyl group at the end of the 5-carbon chain makes the parent chain (the longest continuous chain of carbon atoms) 6 carbons long.
While on the topic of numbering and rookie mistakes, another popular mistake when applying the numbers to the names of compounds is to forget the rule that the LOWEST number possible for designating a group on an alkane must always be used in the name. For example,
Figure 1.1-2 The Lowest Number Possible is Always Used for Alkanes
the rookie mistake would be to name the compound 4-methylpentane.
When more than one alkyl group is attached to the parent chain, the groups are listed alphabetically. Also, if more than one of the same branch are present, the greek prefixes from Tabile 1.1-6 are applied.
Table 1.1-6 Prefixes for Indicating the Number of Identical Groups
| Prefix | Number |
|---|---|
| di- | 2 |
| tri- | 3 |
| tetra- | 4 |
| penta- | 5 |
| hexa- | 6 |
| hepta- | 7 |
| octa- | 8 |
| nona- | 9 |
| deca- | 10 |
| undeca- | 11 |
| dodeca- | 12 |
See the examples below:
Figure 1.1-3 The Lowest Number Possible is Always Used for Alkanes

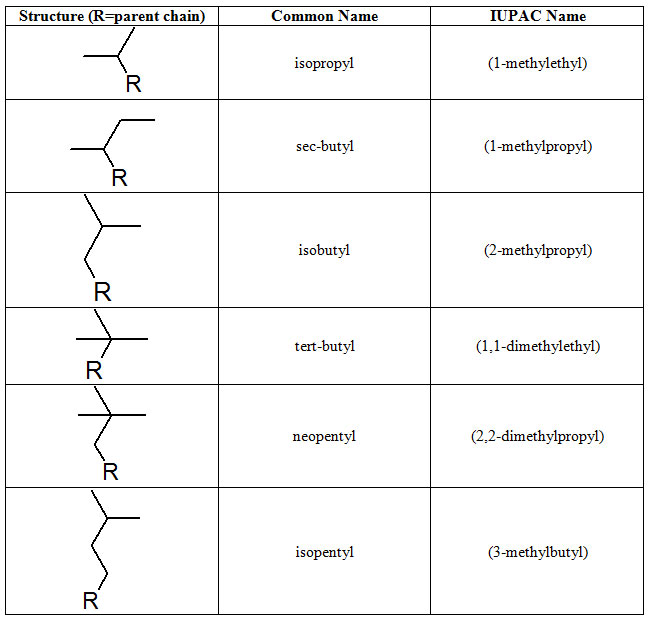
The alkyl groups can be larger than three carbons and there is the additional complication that so many names are "common." By common we mean the names everyone uses for these compounds. Although most common nomenclature is acceptable by the IUPAC, it is not systematic. IUPAC has systematic names for the alkyl groups and several frequently encountered groups appear in table 1.1-7.
Table 1.1-7 More Common and IUPAC names for Branches
Structural Isomers
As you dive deeper into chemistry, you'll find several different types of isomers. Structural isomers (sometimes called constitutional isomers) are different compounds that have the same molecular formula but a different connectivity of the atoms. The compounds in figure 4.25 represent some structural isomers with the molecular formula C6H14. The compounds in figure 4.27 represent a pair of structural isomers with the molecular formula C8H18.
Prefixes Indicating the Presence of a Halogen
The prefixes fluoro-, chloro-, bromo- and iodo- are used to indicate the substitution of a halogen atom for one of the hydrogens on the carbon chain. These compounds are sometimes called alkyl halides or haloalkanes.
Figure 1.1-4 Some Alkyl Halides
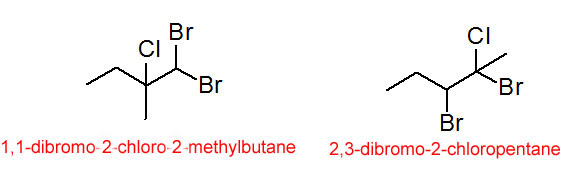
(Note that the compounds in figure 1.1-4 represent a pair of structural isomers.)



