This is "Week 1", section 1.4 from the book 32 Weeks of OChem (v. 1.0).
1.4 Biological Applications
Learning Objectives
- To memorize the nomenclature and structures of the 20 amino acids used for protein synthesis.
- To memorize the pKa's of the ionizable groups on amino acids.
Biochemistry is the application of the chemistry you have learned in general chemistry and will learn in organic chemistry to biological systems. MCAT 2015 has motivated chemical educators to include more biological applications in earlier coursework rather than holding it for the 300-400 level courses. Over these 32 weeks we will be embracing this change with gusto. The place to start the story of biochemistry is with proteins. This week we will look at some of the amino acids used in our cells to make proteins.
The simplest amino acid is glycine.
Figure 1.4-1 The Simplest Amino Acid
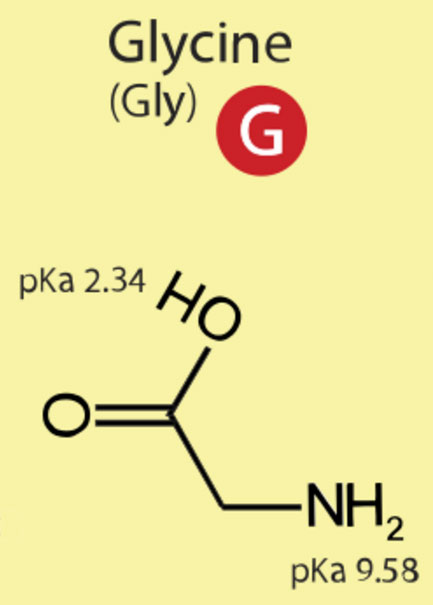
Image Credit:By Dancojocari [CC BY-SA 3.0 (http://creativecommons.org/licenses/by-sa/3.0) or GFDL (http://www.gnu.org/copyleft/fdl.html)], via Wikimedia Commons
In the collection of trillions of cells called you, about 30,000 different kinds of proteins are expressed from your DNA. These tens of thousands of proteins are polymers of just 20 different amino acids. Many of these early biochemistry sections are just asking you to memorize the structures and nomenclature (name, including one-letter and three-letter abbreviations) of these twenty amino acids. As for the pKa's, it's quite satisfactory to do some judicious rounding. Perhaps all the way to the integer number. There is no reason to feel overwhelmed. This week it's just five amino acids. Learn glycine first. Then the only thing that changes for the next four amino acids is the identity of the side-chain that replaces a hydrogen on the "alpha" carbon. The "alpha" carbon,, Cα, is the carbon adjacent to the carbon double bonded to oxygen. The C=O unit moeity is called a carbonyl group.
Figure 1.4-2 More amino acids
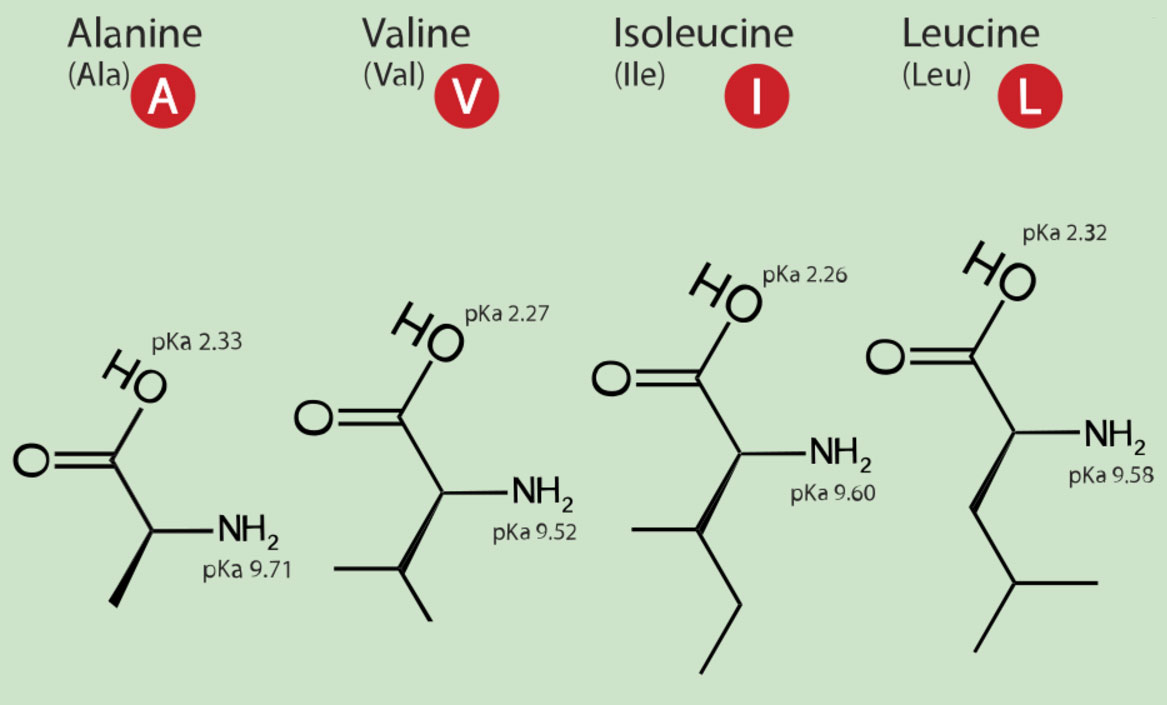
Image Credit:By Dancojocari [CC BY-SA 3.0 (http://creativecommons.org/licenses/by-sa/3.0) or GFDL (http://www.gnu.org/copyleft/fdl.html)], via Wikimedia Commons




