This is "Unit 1", section 1.2 from the book General Chemistry (v. 1.0).
1.2 Chemistry and Matter
Learning Objective
- To be able to define matter.
- To be able to classify matter.
- To be able to define mass.
- To be able to distinguish physical change from chemical change.
- To be able to distinguish chemistry from the other sciences.
- To be aquainted with the different types of chemists.
What do chemists do all day? What exactly do they study? By the end of this section you should be able to answer those questions.
The Study of Matter
Chemists study the structures, physical properties, and chemical properties of material substances. These consist of matterAnything that occupies space and has mass., which is anything that occupies space and has mass. Gold and iridium are matter, as are peanuts, people, and postage stamps. Smoke, smog, and laughing gas are matter. Energy, light, and sound, however, are not matter; ideas and emotions are also not matter.
Figure 1.2(a) An 'Oval Charge'

A Uganda People's Defense Force soldier detonates an 'oval charge' on desired entry point while the team seeks cover around the corner of the building. This type of charge is used to create a hole through a wall when there is no available entrance or for the element of surprise. The Marines and UPDF improved breaching capabilities as they prepare for their African Union Mission in Somalia (AMISOM). Taken on 7 December 2015. Image Credit: By U.S. Department of Defense Current Photos Cpl. Olivia McDonald/U.S. Marine Corps Forces Europe (151126-M-VS306-377) [Public domain], via Wikimedia Commons
Oftentimes, the definition, "Chemistry: The Study of Matter" is lengthened with "...and the changes it undergoes." Those last 5 additional words, though sometimes having some pretty dramatic implications, see the photo above in figure 1.2a, can be omitted for simplicitly and left as understood. The picture above reminds me of many general chemistry students' desire to "blow something up." My recommendation to those students is to join the army. Perhaps more exciting to a chemistry student living in Northwest Illinois is the photograph in figure 1.2b (below).
Figure 1.2(b) The Mississippi River

Matter for chemists to study is everywhere. From the water and all the organisms in the river, to the trees and their solar panels, to the diesel-electric locomotive to the steel in the tracks, to the bridge across the river. Photo taken at Lookout Point in the Palisades State Park in Savanna, IL. If you haven't been there, you need to go. Image credit: By Djngsf (Own work) [CC BY-SA 4.0 (http://creativecommons.org/licenses/by-sa/4.0)], via Wikimedia Commons
The massA fundamental property that does not depend on an object’s location; it is the quantity of matter an object contains. of an object is the quantity of matter it contains. Do not confuse an object's mass with its weightA force caused by the gravitational attraction that operates on an object. The weight of an object depends on its location (c.f. mass)., which is a force caused by the gravitational attraction that operates on the object. Mass is a fundamental property of an object that does not depend on its location. Weight, on the other hand, depends on the location of an object. An astronaut whose mass is 95 kg weighs about 210 lb on Earth but only about 35 lb on the moon because the gravitational force he or she experiences on the moon is approximately one-sixth the force experienced on Earth. For practical purposes, weight and mass are often used interchangeably in laboratories. Because the force of gravity is considered to be the same everywhere on Earth's surface, 2.2 lb (a weight) equals 1.0 kg (a mass), regardless of the location of the laboratory on Earth.
Under normal conditions, there are three distinct states of matter: solids, liquids, and gases (Figure 1.2(c) "The Three States of Matter"). SolidsOne of three distinct states of matter that, under normal conditions, is relatively rigid and has a fixed volume. are relatively rigid and have fixed shapes and volumes. A rock, for example, is a solid. In contrast, liquidsOne of three distinct states of matter that, under normal conditons, has a fixed volume but flows to assume the shape of its container. have fixed volumes but flow to assume the shape of their containers, such as a beverage in a can. GasesOne of three distinct states of matter that, under normal conditions, has neither a fixed shape nor a fixed volume and expands to completely fill its container., such as air in an automobile tire, have neither fixed shapes nor fixed volumes and expand to completely fill their containers. Matter can often change from one physical state to another in a process called a physical changeA change of state that does not affect the chemical composition of a substance.. For example, liquid water can be heated to form a gas called steam, or steam can be cooled to form liquid water. However, such changes of state do not affect the chemical composition of the substance.
Figure 1.2(c) The Three States of Matter

Solids, like the copper wire in the flask above, have a defined shape and volume. Liquids, like the blue solution (copper(II) nitrate and nitric acid) in the flask above, have a fixed volume but flow to assume the shape of their containers. Gases, like the nitrogen dioxide gas in the flask above, completely fill their containers, regardless of volume. Image Credit: By No machine-readable author provided. The mad scientist~commonswiki assumed (based on copyright claims). [Public domain], via Wikimedia Commons
Video: Balls in a box represent atoms or molecules, the particles of matter. The box is shaken to add energy to the system. Low energy shakes give a representation of the particles in the solid state. As the shaking becomes more vigorous, the physical state represented transitions first to a liquid state, where the balls roll over each other, to the gaseous state, where the balls bounce off each other and out of the box. The video concludes with a visualization of a distillation process of a mixture of ethanol and water. Video Credit: Part of NCSSM CORE collection: http://www.dlt.ncssm. Please attribute this work as being created by the North Carolina School of Science and Mathematics. This work is licensed under Creative Commons CC-BY https://creativecommons.org/licenses/by/3.0/ via YouTube
Pure Substances and Mixtures
A pure chemical substance is any matter that has a fixed chemical composition and characteristic properties. Oxygen, for example, is a pure chemical substance that is a colorless, odorless gas at 25°C (room temperature). Very few samples of matter consist of pure substances; instead, most are mixturesA combination of two or more pure substances in variable proportions in which the individual substances retain their respective identities., which are combinations of two or more pure substances in variable proportions in which the individual substances retain their identity. Air, tap water, milk, blue cheese, bread, and dirt are all mixtures. If all portions of a material are in the same state, have no visible boundaries, and are uniform throughout, then the material is homogeneousA mixture in which all portions of a material are in the same state, have no visible boundaries, and are uniform throughout.. Examples of homogeneous mixtures are the air we breathe and the tap water we drink. Homogeneous mixtures are also called solutions. Thus air is a solution of nitrogen, oxygen, water vapor, carbon dioxide, and several other gases; tap water is a solution of small amounts of several substances in water. The specific compositions of both of these solutions are not fixed, however, but depend on both source and location; for example, the composition of tap water in Boise, Idaho, is not the same as the composition of tap water in Buffalo, New York.
If the composition of a material is not completely uniform, then it is heterogeneousA mixture in which a material is not completely uniform throughout. (e.g., chocolate chip cookie dough, blue cheese, and dirt). Mixtures that appear to be homogeneous are often found to be heterogeneous after microscopic examination. Milk, for example, appears to be homogeneous, but when examined under a microscope, it clearly consists of tiny globules of fat and protein dispersed in water (Figure 1.2(d) "A Heterogeneous Mixture"). The components of heterogeneous mixtures can usually be separated by simple means. Solid-liquid mixtures such as sand in water or tea leaves in tea are readily separated by filtration, which consists of passing the mixture through a barrier, such as a strainer, with holes or pores that are smaller than the solid particles. In principle, mixtures of two or more solids, such as sugar and salt, can be separated by microscopic inspection and sorting. More complex operations are usually necessary, though, such as when separating gold nuggets from river gravel by panning. First solid material is filtered from river water; then the solids are separated by inspection. If gold is embedded in rock, it may have to be isolated using chemical methods.
Figure 1.2(d) A Heterogeneous Mixture

Under a microscope(inset and subinset), whole milk is actually a heterogeneous mixture composed of globules of fat and protein dispersed in water. Picture credit: By Internet Archive Book Images [No restrictions], via Wikimedia Commons and Milk Glass: Stefan Kühn (Own work) [GFDL (http://www.gnu.org/copyleft/fdl.html), CC-BY-SA-3.0 (http://creativecommons.org/licenses/by-sa/3.0/) or CC BY-SA 2.5-2.0-1.0 (http://creativecommons.org/licenses/by-sa/2.5-2.0-1.0)], via Wikimedia Commons
Homogeneous mixtures (solutions) can be separated into their component substances by physical processes that rely on differences in some physical property, such as differences in their boiling points.
Most mixtures can be separated into pure substances, which may be either elements or compounds. An elementA pure substance that cannot be broken down into a simpler substance by chemical changes., such as gray, metallic sodium, is a substance that cannot be broken down into simpler ones by chemical changes.
Figure 1.2(e) Sodium metal

Sodium metal is very soft. One can cut it with a butter knife, or even a tablespoon. Picture credit: By The original uploader was Dnn87 at English Wikipedia [CC BY-SA 3.0 (http://creativecommons.org/licenses/by-sa/3.0)], via Wikimedia Commons
A compoundA pure substance that contains two or more elements and has chemical and physical properties that are usually different from those of the elements of which it is composed., such as white, crystalline sodium chloride, contains two or more elements and has chemical and physical properties that are usually different from those of the elements of which it is composed. With only a few exceptions, a particular compound has the same elemental composition (the same elements in the same proportions) regardless of its source or history. The chemical composition of a substance is altered in a process called a chemical changeA process in which the chemical composition of one or more substances is altered.. The conversion of two or more elements, such as sodium and chlorine, to a chemical compound, sodium chloride, is an example of a chemical change, often called a chemical reaction. Currently, 118 elements are known, but millions of chemical compounds have been prepared from these 118 elements. The known elements are listed in the periodic table (see Appendix H: Periodic Table of Elements).You can always acess this periodic table by clicking on the link in the navigation bar at the top of these pages.
In general, a reverse chemical process breaks down compounds into their elements. For example, water (a compound) can be decomposed into hydrogen and oxygen (both elements) by a process called electrolysis. In electrolysis, electricity provides the energy needed to separate a compound into its constituent elements (Figure 1.2(f) "The Decomposition of Water to Hydrogen and Oxygen by Electrolysis"). A similar technique is used on a vast scale to obtain pure aluminum, an element, from its ores, which are mixtures of compounds. Because a great deal of energy is required for electrolysis, the cost of electricity is by far the greatest expense incurred in manufacturing pure aluminum. Thus recycling aluminum is both cost-effective and ecologically sound.
Figure 1.2(f) The Decomposition of Water to Hydrogen and Oxygen by Electrolysis

Water is a chemical compound; hydrogen and oxygen are elements. Bubbles of hydrogen form at the negative battery terminal while bubbles of oxygen form at the positive terminal. Image Credit: "Battery Submerged" by Paul Anderson, https://creativecommons.org/licenses/by/2.0/ via flickr, Molecular Representations and annotations by Highland Community College, Freeport IL.
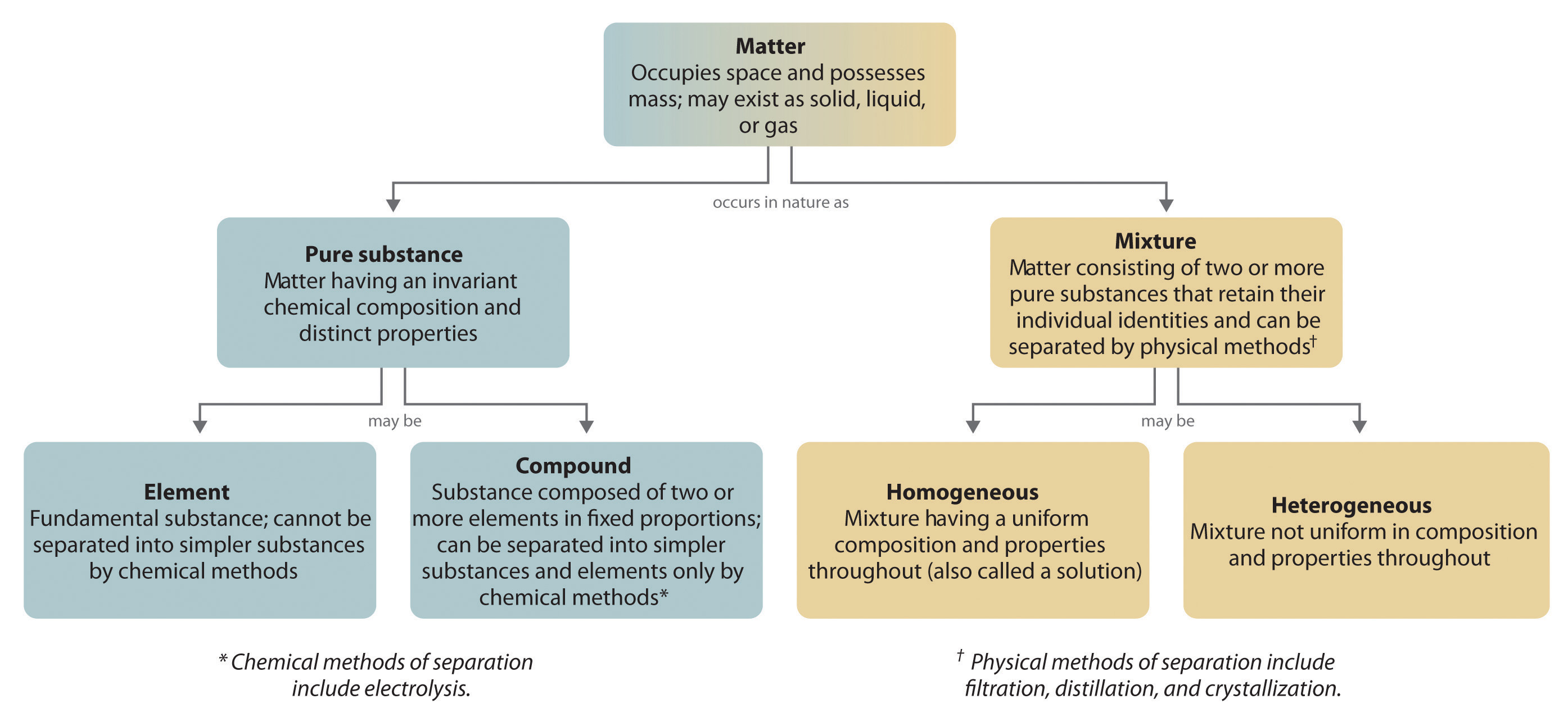
The overall organization of matter and the methods used to separate mixtures are summarized in Figure 1.2(g) "Relationships between the Types of Matter and the Methods Used to Separate Mixtures".
Figure 1.2(g) Relationships between the Types of Matter and the Methods Used to Separate Mixtures
Example 1.2-1
Identify each substance as a compound, an element, a heterogeneous mixture, or a homogeneous mixture (solution).
- filtered tea
- freshly squeezed orange juice
- a compact disc
- aluminum oxide, a white powder that contains a 2:3 ratio of aluminum and oxygen atoms
- selenium
Given: a chemical substance
Asked for: its classification
Strategy:
Step 1 Decide whether a substance is chemically pure. If it is pure, the substance is either an element or a compound. If a substance can be separated into its elements, it is a compound.
Step 2 If a substance is not chemically pure, it is either a heterogeneous mixture or a homogeneous mixture. If its composition is uniform throughout, it is a homogeneous mixture.
Solution:
- Step 1 Tea is a solution of compounds in water, so it is not chemically pure. It is usually separated from tea leaves by filtration. Step 2 Because the composition of the solution is uniform throughout, it is a homogeneous mixture.
- Step 1 Orange juice contains particles of solid (pulp) as well as liquid; it is not chemically pure. Step 2 Because its composition is not uniform throughout, orange juice is a heterogeneous mixture.
- Step 1 A compact disc is a solid material that contains more than one element, with regions of different compositions visible along its edge. Hence a compact disc is not chemically pure. Step 2 The regions of different composition indicate that a compact disc is a heterogeneous mixture.
- Step 1 Aluminum oxide is a single, chemically pure compound.
- Step 1 Selenium is one of the known elements.
Exercise:
Identify each substance as a compound, an element, a heterogeneous mixture, or a homogeneous mixture (solution).
- white wine
- mercury
- ranch-style salad dressing
- table sugar (sucrose)
Answer:
- solution
- element
- heterogeneous mixture
- compound
Properties of Matter
All matter has physical and chemical properties. Physical propertiesA characteristic that scientists can measure without changing the composition of a sample under study. are characteristics that scientists can measure without changing the composition of the sample under study, such as mass, color, and volume (the amount of space occupied by a sample). Chemical propertiesThe characteristic ability of a substance to react to form new substances. describe the characteristic ability of a substance to react to form new substances; they include its flammability and susceptibility to corrosion. All samples of a pure substance have the same chemical and physical properties. For example, pure copper is always a reddish-brown solid (a physical property) and always dissolves in dilute nitric acid to produce a blue solution and a brown gas (a chemical property).
Branches of Science
Recall from the previous section that we defined science as a way of knowing. Now in this section we have defined chemistry as the study of matter. Where does chemistry fit in the scheme of science?
Figure 1.2(h) Branches of Science

As an introduction to science, the Scale of the Universe is mapped to the Branches of Science. The Scale of the Universe captures the average diameter of key systems. The scale needs to be cubed to make volume; it takes about 60 trillion atoms to make a human cell, 100 trillion cells to make a person, and 108 billion people have lived to build modern society. Branches of Science captures the major areas of study in science. The images capture the building block for the scientific branch. Math is built up from binary logic, physics is built on atomic scale physical laws, life science is built by cellular behavior calculated through natural selection, social science is built on the human brain, and earth and space science begins with our own planetary body. One to many bullet point graphics plus visual stacking show how scientific systems are built one atop the next. Mathematics is groups of logical units, chemistry is groups of atoms following physical laws, functional biology is groups of cells functioning as organisms and ecosystems, and sociology is groups of people governed by their individual psychology. Picture credit: By Efbrazil (Own work) [CC BY-SA 3.0 (http://creativecommons.org/licenses/by-sa/3.0)], via Wikimedia Commons
A popular general chemistry textbook is titled "Chemistry: The Central Science." The author's point being that all sciences use fundamental concepts from general chemistry to explain their particular branch of science. This isn't to say chemistry is the most important science, but instead chemistry is the only science used by all the other sciences. Chemistry is central because all sciences involve the study of some kind of matter. Whether that matter is living or dead, planetary or submicroscopic, if it has volume and mass, it's something understood at a molecular level using the chemistry concepts you will learn in this course.
Branches of Chemistry
Chemistry is composed of incredibly diverse branches. To give you some idea a collection of links to Wikipedia is provided below. Realize that a chemist is not necessarily limited to one particular branch. There's lots of overlap. I remember when I was trying to figure out what kind of chemist I was, I found myself torn between biochemistry and analytical chemistry. Aware of my dilema, a very senior biochemist in our department pointed out to me that "We're all analytical chemists." As I learn more chemistry, I find more and more overlap between the branches.
Summary
Chemistry is the study of matter. Matter is anything that occupies space and has mass. The three states of matter are solid, liquid, and gas. A physical change involves the conversion of a substance from one state of matter to another, without changing its chemical composition. Most matter consists of mixtures of pure substances, which can be homogeneous (uniform in composition) or heterogeneous (different regions possess different compositions and properties). Pure substances can be either chemical compounds or elements. Compounds can be broken down into elements by chemical reactions, but elements cannot be separated into simpler substances by chemical means. The properties of substances can be classified as either physical or chemical. Scientists can observe physical properties without changing the composition of the substance, whereas chemical properties describe the tendency of a substance to undergo chemical changes (chemical reactions) that change its chemical composition. Chemistry is an important tool for all scientific disciplines. There are many specializations from which chemists can choose.
Key Takeaway
- Matter can be classified according to physical and chemical properties.





