This is "Unit 1", section 1.1 from the book General Chemistry (v. 1.0).
1.1 Chemistry and Science
Learning Objectives
- To be able to recognize science as one way of knowing.
- To know three criteria that distinguish science from other ways of knowing.
- To establish a historical framework for science beginning with Galileo.
- To identify the components of the scientific method.
What is science? You probably have some notion, but do you have a clear and concise set of critieria that distinguish a scientific discipline from a non-scientific pursuit? Are engineers scientists? What about medical doctors? Arguably, all of humanity has been doing some aspects of science for as long as we've been on the planet. And finally. what does it mean "to do science?" In this text, we will give some historical background that will give you a framework for appreciating what we call science and introduce some of the key participants in this continuously expanding endeavor.
Ways of Knowing
Figure 1.1(a) Mona Lisa, by Leonardo da Vinci, 1506

Leonardo da Vinci [Public domain or Public domain], via Wikimedia Commons
Art is a way of knowing. We've all seen pictures of the Mona Lisa. For some people, when they look at the Mona Lisa, they are able to visually know something about the feminine mystique. Or maybe when they see the Mona Lisa, they know what she is feeling. Personally, I know a feeling of contentedness and satisfaction when I look at the Mona Lisa that I just can't put into words. That's what's so wonderful about art. It's a way of knowing feelings. Science is really lousy for knowing feelings. Science, however, is unparalleled for knowing nature and how nature behaves.
Science is a way of knowing which has three requirements.
- Reproducible observation
- Controlled experiment
- Theory based upon mathematical reasoning.
By reproducible, we mean miracles can not be explained by science. Reproducible means the observation can be made any time and any place given the appropriate circumstances. As an illustration of how science works consider the case for cold fusion. In the late 1980's, fresh from an energy crisis in 1973, a group of researchers announced with great fanfare a nuclear process that turned two hydrogen atoms into one helium atom and gave energy as a product. They claimed they could do this at room temperature using a special catalyst and called their process cold fusion. Of course everyone was very excited, but when other scientists tried to reproduce the researchers observation, none were able to do so. Since then cold fusion has become something of a joke in the scientific community.
Controlled experiments are those that can be designed with a control. By that we mean we can manipulate the reaction conditions such that the some variables can be changed in one experiment while being held the same in another experiment. The experiment where the variable is unchanged is called the control.
The mathematical theory involves an equation, perhaps even a fundamental equation like Newton's second law, F = ma. Recall, Newton's second law says force and acceleration are directly proportional by a factor called mass. Alternatively you could interpret the equation as stating force and mass are directly proportional by a factor called acceleration. Still another way of stating Newton's second law is to say mass is inversely proportional to acceleration. The bigger the mass, the less the object will be accelerated by a given force. There are many kinds of mathematical relationships. In this course most of our relationships will be direct or inverse. Instead of memorizing equations, scientists are trained to think about relationships.
Figure 1.1(b) Low Energy Nuclear Reactions (LENR)
Image credit: By LoKiLeCh (Own work) [CC BY-SA 3.0 (http://creativecommons.org/licenses/by-sa/3.0)], via Wikimedia Commons
The Scientific Method
Figure 1.1(c) The Scientific Method
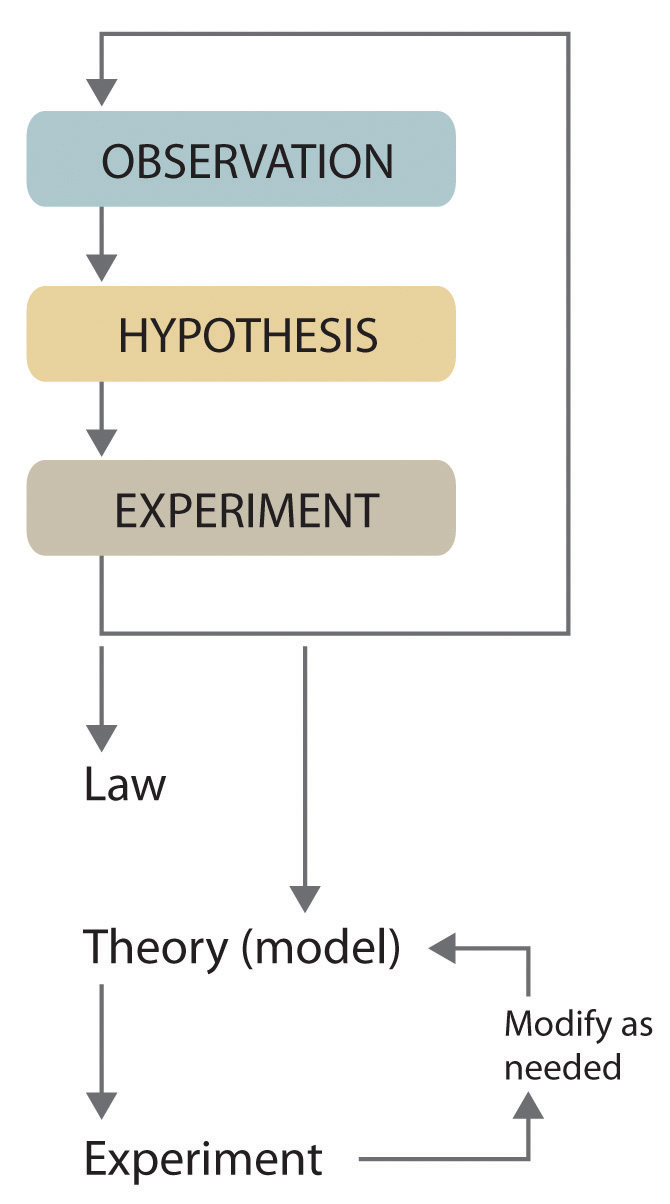
Looking at the diagram in figure 1.1(c) above, one might come away with the idea that the scientific method is a linear process with a beginning, a middle and an end. Look closer and you see it's actually representing a pair of circles, one circle generating another circle. In fact, the scientific method is much more complex and evolving than this schematic implies. Each circle can generate literally thousands of other circles. And each of those circles can generate literally thousands more circles and so forth. Science is never done. Science doesn't have an end and it has unlimited beginnings. The life's work of a scientist is often one tiny piece of one small circle. Most of people working in science grind away in the colored boxes of the top cycle-observation, hypothesis, experiment. Their work is guided by a senior investigator feeding junior team members hypotheses, making suggestions for experiments and mulling results. Junior researchers become more and more involved in the process and before they know it, the researcher is on the way to becoming what is called a principle investigator (PI). You know, like this guy
Figure 1.1(d)Magnum PI

Image Credit: photo by Alan Light [CC BY 2.0 (http://creativecommons.org/licenses/by/2.0)], via Wikimedia Commons
But seriously, some of the terms in Figure 1.1c deserve a little explanation:
- Observation: Can be quantitatve or qualitive. The next section of the text looks at how scientists make quantitative observations. Scientists are required to follow special rules associated with making quantitative observations.
- Hypothesis: A guess as to what's going on. Sometimes people don't like to hear that scientists make guesses, but that's really what it's all about. Hypothetical statements often start with "I wonder if...."
- Experiment: Inspired by hypothesis and designed to facilitate observation.
- Law: Nature's promise about what will happen. These promises are NEVER broken and have no exceptions.
- Theory: Man's explanation about why something happens.
The Story of Science
Figure 1.1(e) Galileo Galilei (1564-1642)

Image Credit: Peter Paul Rubens [Public domain], via Wikimedia Commons
"Philosophy is written in this grand book — I mean the universe — which stands continually open to our gaze, but it cannot be understood unless one first learns to comprehend the language in which it is written. It is written in the language of mathematics, and its characters are triangles, circles, and other geometric figures, without which it is humanly impossible to understand a single word of it; without these, one is wandering about in a dark labyrinth." Galileo Galilei, The Assayer, 1623.
It's difficult to say where the story of science starts. It probably started when we were discovering fire, but as a formal discipline, a lot of credit needs to be given to Galileo. He wrote the statement above even before the word science had been invented. So where he wrote "philosophy," you can read science. Notice how well that quote matches our defining characteristics of what we call science.
Reproducible observation and controlled experiment = continually open to our gaze
Mathematical reasoning = written in the language of mathematics
The starting point of our story of science is a little vague, but the dates of Galileo's time on the planet are worth remembering. Particularly the date of his death, 1642, as it is the year Isaac Newton was born.
Key Takeaway
- Scientists have been using the scientific method for hundreds, if not thousands, of years as a way of knowing about nature.




