This is "Unit 12", section 12.3 from the book General Chemistry (v. 1.0).
12.3 Dalton's Law of Partial Pressures
Learning Objective
- To determine the contribution of each component gas to the total pressure of a mixture of gases.
One of the properties of gases is that they mix with each other. When they do so, they become a solution-a homogeneous mixture. Some of the properties of gas mixtures are easy to determine if we know the composition of the gases in the mix.
In gas mixtures, each component in the gas phase can be treated separately. Each component of the mixture shares the same temperature and volume. (Remember that gases expand to fill the volume of their container; gases in a mixture continue to do that as well.) However, each gas has its own pressure. The partial pressureThe pressure that an individual gas in a mixture has. of a gas, Pi, is the pressure that an individual gas in a mixture has. Partial pressures are expressed in torr, millimeters of mercury, or atmospheres like any other gas pressure; however, we use the term pressure when talking about pure gases and the term partial pressure when we are talking about the individual gas components in a mixture.
To summarize, the total pressure exerted by a mixture of gases is the sum of the partial pressures of component gases. This law was first discovered by John Dalton, the father of the atomic theory of matter. It is now known as Dalton’s law of partial pressuresA law that states that the total pressure exerted by a mixture of gases is the sum of the partial pressures of component gases.. We can write it mathematically as
Equation 12.3(eq1)
where Pt is the total pressure and the other terms are the partial pressures of the individual gases (Figure 12.3(a)).
Figure 12.3(a) Dalton’s Law
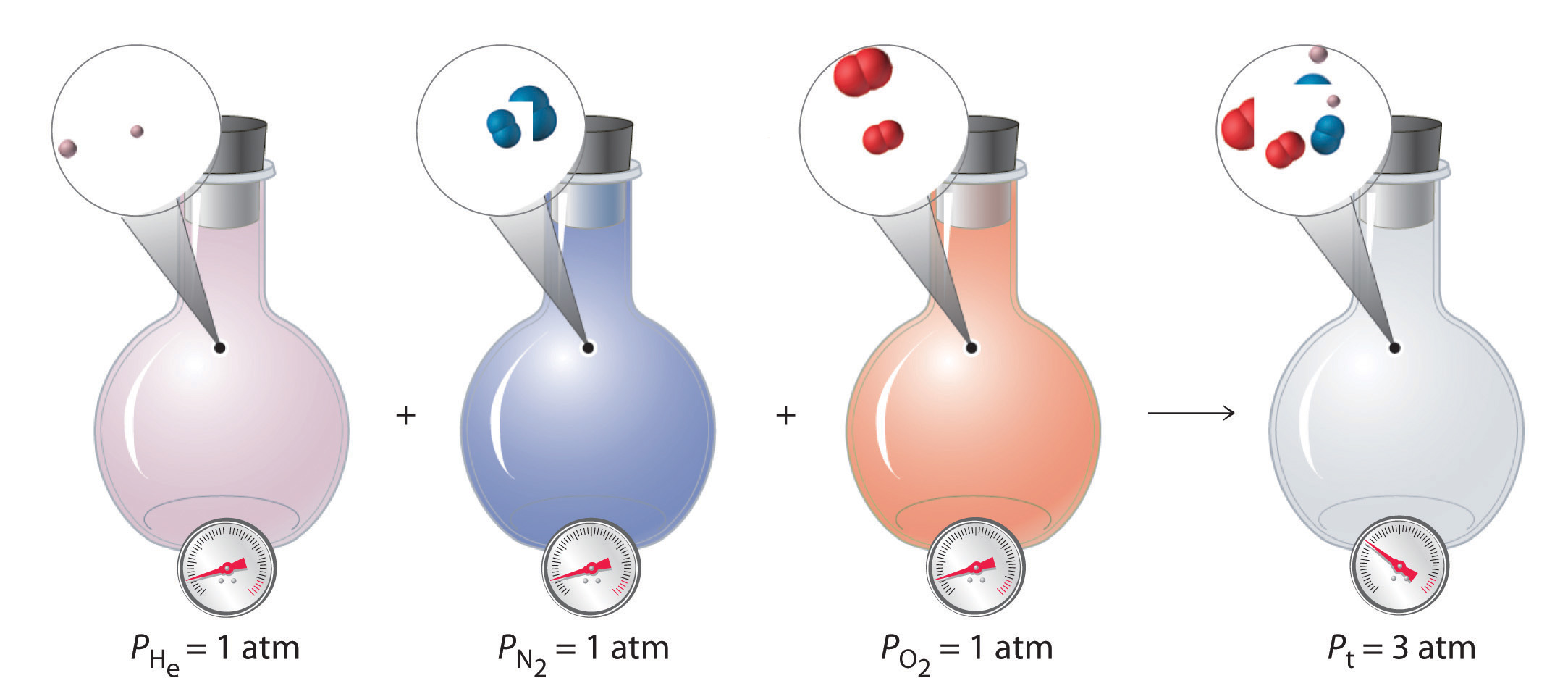
The total pressure of a mixture of gases is the sum of the partial pressures of the individual gases.
Although this may seem to be a trivial law, it reinforces the idea that gases behave independently of each other.
Example 12.3-1
A mixture of H2 at 2.33 atm and N2 at 0.77 atm is in a container. What is the total pressure in the container?
Solution
Dalton's law of partial pressures states that the total pressure is equal to the sum of the partial pressures. We simply add the two pressures together:
Ptot = 2.33 atm + 0.77 atm = 3.10 atmExercise
Air can be thought of as a mixture of N2 and O2. In 760 torr of air, the partial pressure of N2 is 608 torr. What is the partial pressure of O2?
Answer
152 torr
Example 12.3-2
A 2.00 L container with 2.50 atm of H2 is connected to a 5.00 L container with 1.90 atm of O2 inside. The containers are opened, and the gases mix. What is the final pressure inside the containers?
Solution
Because gases act independently of each other, we can determine the resulting final pressures using Boyle's law and then add the two resulting pressures together to get the final pressure. The total final volume is 2.00 L + 5.00 L = 7.00 L. First, we use Boyle's law to determine the final pressure of H2:
Create a table of values from the problem.
| V1 = 2.00 L | V2 = 7.00 L |
| P1 = 2.50 atm | P2 = ? |
From Boyle's Law, since the volume is increasing, the pressure must be decreasing. Therefore the volume factor must be less than one. In other words, the smaller volume must go on top of the bigger volume; otherwise the volume factor would be more than one.
Now we do that same thing for the O2:
Create a table of values from the problem.
| V1 = 5.00 L | V2 = 7.00 |
| P1 = 1.90 atm | P2 = ? |
From Boyle's Law, since the volume is increasing, the pressure must be decreasing. Therefore the volume factor must be less than one. In other words, the smaller volume must go on top of the bigger volume; otherwise the volume factor would be more than one.
The total pressure is the sum of the two resulting partial pressures:
Ptot = 0.714 atm + 1.36 atm = 2.07 atmExercise
If 0.75 atm of He in a 2.00 L container is connected to a 3.00 L container with 0.35 atm of Ne and the containers are opened, what is the resulting total pressure?
Answer
0.51 atm
One of the reasons we have to deal with Dalton's law of partial pressures is because gases are frequently collected by bubbling through water. As we will see in the next chapter, liquids are constantly evaporating into a vapor until the vapor achieves a partial pressure characteristic of the substance and the temperature. This partial pressure is called a vapor pressureThe partial pressure exerted by evaporation of a liquid.. Table 12.3(1) lists the vapor pressures of H2O versus temperature. Note that if a substance is normally a gas under a given set of conditions, the term partial pressure is used; the term vapor pressure is reserved for the partial pressure of a vapor when the liquid is the normal phase under a given set of conditions.
Table 12.3(1) Vapor Pressure of Water versus Temperature
| Temperature (°C) | Vapor Pressure (torr) | Temperature (°C) | Vapor Pressure (torr) | |
|---|---|---|---|---|
| 5 | 6.54 | 30 | 31.84 | |
| 10 | 9.21 | 35 | 42.20 | |
| 15 | 12.79 | 40 | 55.36 | |
| 20 | 17.54 | 50 | 92.59 | |
| 21 | 18.66 | 60 | 149.5 | |
| 22 | 19.84 | 70 | 233.8 | |
| 23 | 21.08 | 80 | 355.3 | |
| 24 | 22.39 | 90 | 525.9 | |
| 25 | 23.77 | 100 | 760.0 |
Any time a gas is collected over water, the total pressure is equal to the partial pressure of the gas plus the vapor pressure of water. This means that the amount of gas collected will be less than the total pressure suggests.
Example 12.3-3
Hydrogen gas is generated by the reaction of nitric acid and elemental iron. The gas is collected in an inverted 2.00 L container immersed in a pool of water at 22°C. At the end of the collection, the partial pressure inside the container is 733 torr. How many moles of H2 gas were generated?
Solution
We need to take into account that the total pressure includes the vapor pressure of water. According to Table 12.3(1) , the vapor pressure of water at 22°C is 19.84 torr. According to Dalton's law of partial pressures, the total pressure equals the sum of the pressures of the individual gases, so
We solve by subtracting:
Since we are heading to the ideal gas law, we need to convert the pressure units to atm.
Now we can use the ideal gas law to determine the number of moles (remembering to convert temperature to kelvins, making it 295 K):
All the units cancel except for mol, which is what we are looking for. So
n = 0.0775 mol H2 collectedExercise
CO2, generated by the decomposition of CaCO3, is collected in a 3.50 L container over water. If the temperature is 50°C and the total pressure inside the container is 833 torr, how many moles of CO2 were generated?
Answer
0.129 mol
Summary
The pressure exerted by each gas in a gas mixture (its partial pressure) is independent of the pressure exerted by all other gases present. Consequently, the total pressure exerted by a mixture of gases is the sum of the partial pressures of the components (Dalton’s law of partial pressures).
Numerical Problems
-
What is the total pressure of a gas mixture containing these partial pressures: , , and ?
-
What is the total pressure of a gas mixture containing these partial pressures: PNe = 312 torr, PHe = 799 torr, and PAr = 831 torr?
-
In a gas mixture of He and Ne, the total pressure is 335 torr and the partial pressure of He is 0.228 atm. What is the partial pressure of Ne?
-
In a gas mixture of O2 and N2, the total pressure is 2.66 atm and the partial pressure of O2 is 888 torr. What is the partial pressure of N2?
-
A 3.55 L container has a mixture of 56.7 g of Ar and 33.9 g of He at 33°C. What are the partial pressures of the gases and the total pressure inside the container?
-
A 772 mL container has a mixture of 2.99 g of H2 and 44.2 g of Xe at 388 K. What are the partial pressures of the gases and the total pressure inside the container?
-
A sample of O2 is collected over water in a 5.00 L container at 20°C. If the total pressure is 688 torr, how many moles of O2 are collected?
-
A sample of H2 is collected over water in a 3.55 L container at 50°C. If the total pressure is 445 torr, how many moles of H2 are collected?
-
A sample of CO is collected over water in a 25.00 L container at 5°C. If the total pressure is 0.112 atm, how many moles of CO are collected?
-
A sample of NO2 is collected over water in a 775 mL container at 25°C. If the total pressure is 0.990 atm, how many moles of NO2 are collected?
-
A sample of NO is collected over water in a 75.0 mL container at 25°C. If the total pressure is 0.495 atm, how many grams of NO are collected?
-
A sample of ClO2 is collected over water in a 0.800 L container at 15°C. If the total pressure is 1.002 atm, how many grams of ClO2 are collected?
Answers
-
2.70 atm
-
-
162 torr, or 0.213 atm
-
-
PAr = 10.0 atm; PHe = 59.9 atm; Ptot = 69.9 atm
-
-
0.183 mol
-
-
0.113 mol
-
-
0.0440 g
-





