This is "Unit 4", section 4.4 from the book General Chemistry (v. 1.0).
4.4 Names and Formulas of Binary Ionic Compounds: Easy Ones
Learning Objective
- To name ionic compounds.
Many compounds, particularly those that have been known for a relatively long time, have more than one name: a common name (sometimes more than one) and a systematic name, which is the name assigned by adhering to specific rules. Like the names of most elements, the common names of chemical compounds generally have historical origins, although they often appear to be unrelated to the compounds of interest. For example, the systematic name for CaO is calcium oxide, but its common name is lime. In this section we will learn to write systematic names of salts having metals that only have one charge in nature. We will extend the system we learn in this section when we tackle more complicated salts in unit 5.
Figure 4.4(a) Highfield Lime Kiln

Calcium Oxide, commonly known as lime, is an important modern and historical building material. Lime is made by roasting quarried limestone (calcium carbonate) in a kiln. Highfield Lime Kiln, North Ayrshire, Scotland - kiln ruins with the 'eye' exposed. By Rosser1954 (Own work) [CC BY-SA 4.0 (https://creativecommons.org/licenses/by-sa/4.0)], via Wikimedia Commons.
Predicting the Charge on Ions
Consistent with a tendency to have the same number of electrons as the nearest noble gas, when forming ions, elements in groups 1, 2, and 3 tend to lose one, two, and three electrons, respectively, to form cations, such as Na+ and Mg2+. They then have the same number of electrons as the nearest noble gas: neon. Similarly, K+, Ca2+, and Sc3+ have 18 electrons each, like the nearest noble gas: argon. In addition, the elements in group 13 lose three electrons to form cations, such as Al3+, again attaining the same number of electrons as the noble gas closest to them in the periodic table. Conversely, elements in groups 17, 16, and 15 often react to gain one, two, and three electrons, respectively, to form ions such as Cl-, S2-, and P3-. Ions such as these, which contain only a single atom, are called monatomic ionsAn ion with only a single atom.. You can predict the charges of most monatomic ions derived from the main group elements by simply looking at the periodic table and counting how many columns an element lies from the extreme left or right. For example, you can predict that barium (in group 2) will form Ba2+ to have the same number of electrons as its nearest noble gas, xenon, that oxygen (in group 16) will form O2- to have the same number of electrons as neon, and cesium (in group 1) will form Cs+ to also have the same number of electrons as xenon. Additionaly, there are two important transition metals that form ions having only one charge. Silver forms the Ag+ ion and zinc forms the Zn2+ ion. Some common monatomic ions are in Table 4.4(1).
Note the Pattern
Elements in groups 1, 2, and 3 tend to form 1+, 2+, and 3+ ions, respectively; elements in groups 15, 16, and 17 tend to form 3-, 2-, and 1- ions, respectively. Ag forms Ag+ and Zn forms Zn2+.
Table 4.4(1) Some Common Monatomic Ions and Their Names
| Group 1 | Group 2 | ---------- | Group 11 | Group 12 | Group 13 | Group 15 | Group 16 | Group 17 |
|---|---|---|---|---|---|---|---|---|
Li+ lithium |
Be2+ beryllium |
N3- nitride |
O2- oxide |
F- fluoride |
||||
Na+ sodium |
Mg2+ magnesium |
Al3+ aluminum |
P3- phosphide |
S2- sulfide |
Cl- chloride |
|||
K+ potassium |
Ca2+ calcium |
Zn2+ zinc |
As3- arsenide |
Se2- selenide |
Br- bromide |
|||
Rb+ rubidium |
Sr2+ strontium |
Ag+ silver |
Te2- telluride |
I- iodide |
||||
Cs+ cesium |
Ba2+ barium |
Example 4.4-1
Predict the charge on the most common monatomic ion formed by each element.
- aluminum, used in the quantum logic clock, the world's most precise clock
- selenium, used to make ruby-colored glass
Given: element
Asked for: ionic charge
Strategy:
A Identify the group in the periodic table to which the element belongs. Based on its location in the periodic table, decide whether the element is a metal, which tends to lose electrons; a nonmetal, which tends to gain electrons; or a semimetal, which can do either.
B After locating the noble gas that is closest to the element, determine the number of electrons the element must gain or lose to have the same number of electrons as the nearest noble gas.
Solution:
- A Aluminum is a metal in group 13; consequently, it will tend to lose electrons. B The nearest noble gas to aluminum is neon. Aluminum will lose three electrons to form the Al3+ ion, which has the same number of electrons as neon.
- A Selenium is a nonmetal in group 16, so it will tend to gain electrons. B The nearest noble gas is krypton, so we predict that selenium will gain two electrons to form the Se2- ion, which has the same number of electrons as krypton.
Exercise
Predict the charge on the most common monatomic ion formed by each element.
- calcium, used to prevent osteoporosis
- iodine, required for the synthesis of thyroid hormones
Answer:
- Ca2+
- I-
Formulas of Binary Ionic Compounds
An ionic compound that contains only two elements, one present as a cation and one as an anion, is called a binary ionic compoundAn ionic compound that contains only two elements, one present as a cation and one as an anion.. One example is MgCl2, a coagulant used in the preparation of tofu from soybeans. For binary ionic compounds, the subscripts in the empirical formula can also be obtained by crossing charges: use the absolute value of the charge on one ion as the subscript for the other ion. This method is shown schematically as follows:
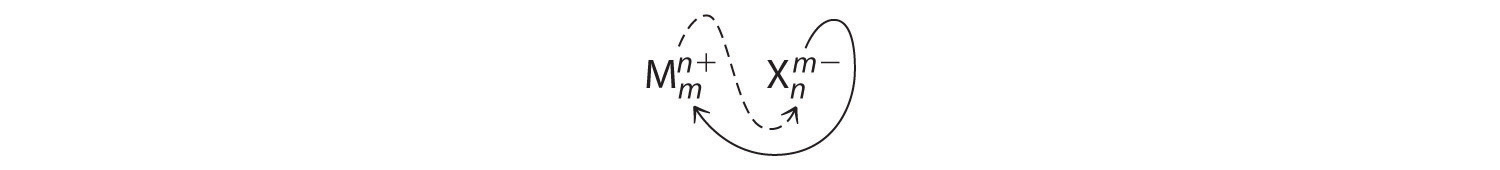
Crossing charges. One method for obtaining subscripts in the empirical formula is by crossing charges.
When crossing charges, you will sometimes find it necessary to reduce the subscripts to their simplest ratio to write the empirical formula. Consider, for example, the compound formed by Mg2+ and O2-. Using the absolute values of the charges on the ions as subscripts gives the formula Mg2O2:
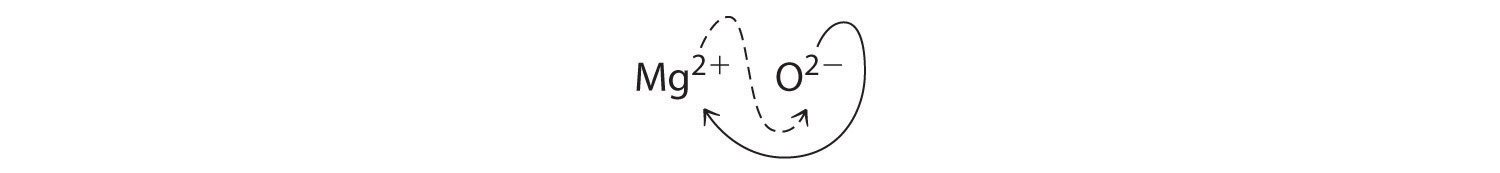
This simplifies to its correct empirical formula MgO. The empirical formula has one Mg2+ ion and one O2- ion.
Example 4.4-2
Write the formula for the simplest binary ionic compound formed from each ion or element pair.
- Ga3+ and As3-
- Eu3+ and O2-
- calcium and chlorine
Given: ions or elements
Asked for: empirical formula for binary ionic compound
Strategy:
A If not given, determine the ionic charges based on the location of the elements in the periodic table.
B Use the absolute value of the charge on each ion as the subscript for the other ion. Reduce the subscripts to the lowest numbers to write the empirical formula. Check to make sure the empirical formula is electrically neutral.
Solution:
B Using the absolute values of the charges on the ions as the subscripts gives Ga3As3:
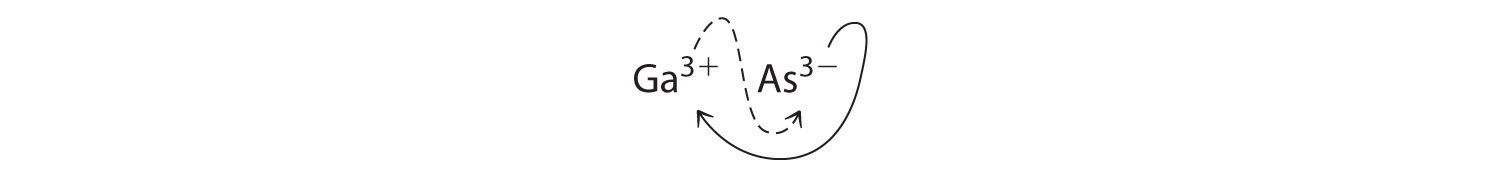
Reducing the subscripts to the smallest whole numbers gives the empirical formula GaAs, which is electrically neutral [+3 + (-3) = 0]. Alternatively, we could recognize that Ga3+ and As3- have charges of equal magnitude but opposite signs. One Ga3+ ion balances the charge on one As3- ion, and a 1:1 compound will have no net charge. Because we write subscripts only if the number is greater than 1, the formula is GaAs. GaAs is gallium arsenide, which is widely used in the electronics industry in transistors and other devices.
B Because Eu3+ has a charge of +3 and O2- has a charge of -2, a 1:1 compound would have a net charge of +1. We must therefore find multiples of the charges that cancel. We cross charges, using the absolute value of the charge on one ion as the subscript for the other ion:
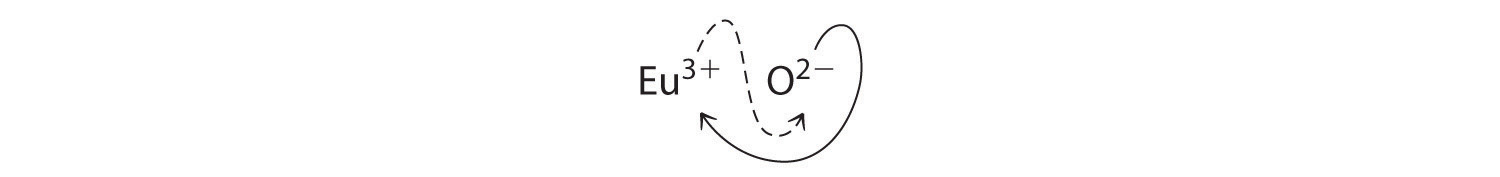
The subscript for Eu3+ is 2 (from O2-), and the subscript for O2- is 3 (from Eu3+), giving Eu2O3; the subscripts cannot be reduced further. The formula contains a positive charge of 2(+3) = +6 and a negative charge of 3(-2) = -6, for a net charge of 0. The compound Eu2O3 is neutral. Europium oxide is responsible for the red color in television and computer screens.
A Because the charges on the ions are not given, we must first determine the charges expected for the most common ions derived from calcium and chlorine. Calcium lies in group 2, so it should lose two electrons to form Ca2+. Chlorine lies in group 17, so it should gain one electron to form Cl-.
B Two Cl- ions are needed to balance the charge on one Ca2+ ion, which leads to the formula CaCl2. We could also cross charges, using the absolute value of the charge on Ca2+ as the subscript for Cl and the absolute value of the charge on Cl- as the subscript for Ca:
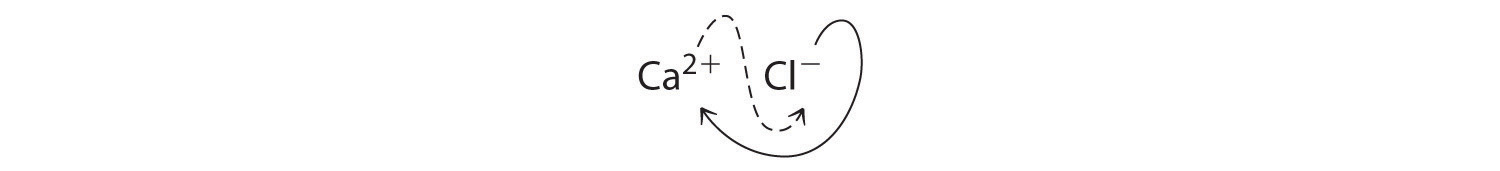
The subscripts in CaCl2 cannot be reduced further. The formula is electrically neutral [+2 + 2(-1) = 0]. This compound is calcium chloride, one of the substances used as "salt" to melt ice on roads and sidewalks in winter.
Exercise
Write the formula for the simplest binary ionic compound formed from each ion or element pair.
- Li+ and N3-
- Al3+ and O2-
- lithium and oxygen
Answer:
- Li3N
- Al2O3
- Li2O
Naming Binary Ionic Compounds
The formulas are highly informative, but they have some disadvantages. First, they are inconvenient for routine verbal communication. For example, saying “C-A-three-P-O-four-two” for Ca3(PO4)2 is much more difficult than saying “calcium phosphate.”
We begin with binary ionic compounds, which contain only two elements. The procedure for naming such compounds is outlined in Figure 4.4(b) and uses the following steps:
Figure 4.4(b) Naming an Ionic Compound
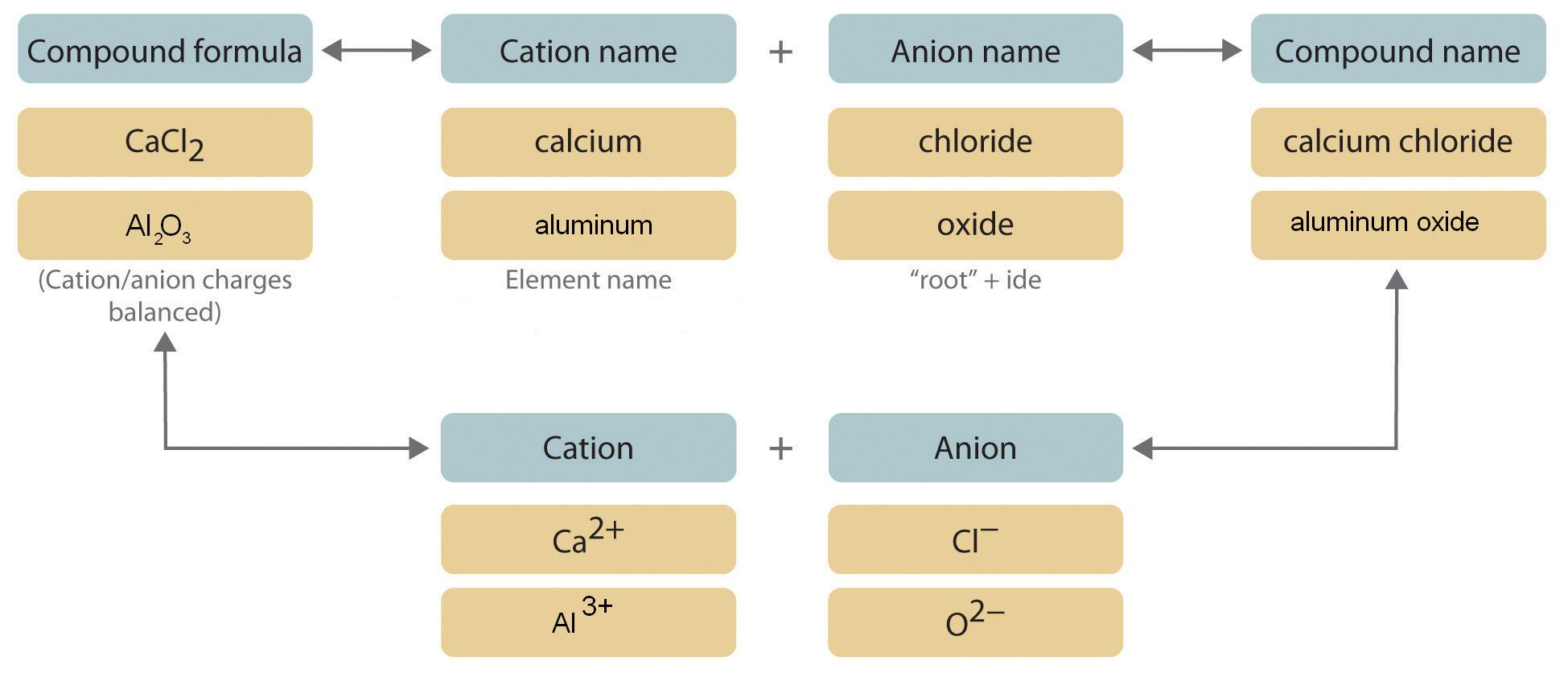
Taking it step-wise through figure 4.4(b):
Name the cation.
The name of the cation of a metal that forms only one cation is the same as the name of the metal (with the word ion added if the cation is by itself). For example, Na+ is the sodium ion, Ca2+ is the calcium ion, and Al3+ is the aluminum ion.Name the anion.
Monatomic anions are named by adding the suffix -ide to the root of the name of the parent element; thus, Cl− is chloride, O2− is oxide, P3− is phosphide, N3− is nitride, and C4− is carbide. Because the charges on these ions can be predicted from their position in the periodic table, it is not necessary to specify the charge in the name. Examples of monatomic anions are in Table 4.4(1) .
Write the name of the compound as the name of the cation followed by the name of the anion.
It is not necessary to indicate the number of cations or anions present per formula unit in the name of an ionic compound because this information is implied by the charges on the ions. You must consider the charge of the ions when writing the formula for an ionic compound from its name, however. Because the charge on the chloride ion is −1 and the charge on the calcium ion is +2, for example, consistent with their positions in the periodic table, simple arithmetic tells you that calcium chloride must contain twice as many chloride ions as calcium ions to maintain electrical neutrality. Thus the formula is CaCl2. The best way to learn how to name ionic compounds is to work through a few examples, referring to Figure 4.4(b) , Table 4.4(1) .
Note the Pattern
Cations are always named before anions.
Example 6
Write the systematic name (and the common name if applicable) for each ionic compound.
- LiCl
- Mg3P2
- ZnBr2
- AlI3
Given: formula
Asked for: name
Strategy:
A Name of the cation, consulting Table 4.4(1).
B Name of the anion, again consulting Table 4.4(1).
C Beginning with the cation, write the name of the compound.
Solution:
- A B Lithium is in group 1, so we know that it forms the Li+ cation, which is the lithium ion. Similarly, chlorine is in group 17, so it forms the Cl− anion, which is the chloride ion. C Because we begin with the name of the cation, the name of this compound is lithium chloride, which is used medically as an antidepressant drug.
- A B The cation is the magnesium ion, and the anion is the phosphide ion. C Because we list the cation first, the name of this compound is magnesium phosphide. Magnesium phosphide is a fumigant for the control of cigarette beetle.
- A B The cation is the zinc ion , and the anion is the bromide ion. C The compound is therefore zinc bromide, which is used to make a transparent radiation shield.
- A B The cation is the aluminum ion. The anion is iodide ion. C The name of this compound is aluminum iodide, which has uses as a reagent in organic synthesis.
Exercise
Write the systematic name (and the common name if applicable) for each ionic compound.
- AgCl
- Na2S
- BaBr2
Answer:
- silver chloride
- sodium sulfide
- barium bromide
Summary
Ionic compounds are named according to systematic procedures, although common names are widely used. Systematic nomenclature enables us to write the structure of any compound from its name and vice versa. Ionic compounds are named by writing the cation first, followed by the anion.
Key Takeaway
- There is a systematic method used to name ionic compounds.
Conceptual Problems
-
Name each cation.
- K+
- Al3+
- Ag+
- Mg2+
- Li+
-
Name each anion.
- Br−
- P3-
- S2−
- F−
-
Name each compound.
- MgBr2
- Na3N
- CaO
- SrCl2
- Ag2S
-
Name each compound.
- Na2O
- CaI2
- K3As
- Li4C
- MgF2
Answers
-
-
-
- magnesium bromide
- sodium nitride
- calcium oxide
- strontium chloride
- silver sulfide
-
- sodium oxide
- calcium iodide
- potassium arsenide
- lithium carbide
- magnesium fluoride
Numerical Problems
-
Predict the charge on the most common monatomic ion formed by each element.
- chlorine
- phosphorus
- scandium
- magnesium
- arsenic
- oxygen
-
Predict the charge on the most common monatomic ion formed by each element.
- sodium
- selenium
- barium
- rubidium
- nitrogen
- aluminum
-
For each ionic compound, name the cation and the anion and give the charge on each ion.
- BeO
- Ag2Se
- BaS
- Zn3P2
- CsF
-
Write the formula for each compound.
- potassium oxide
- silver iodide
- silver sulfide
- barium nitride
- cesium iodide
-
Write the formula for each compound.
- magnesium chloride
- zinc oxide
- potassium fluoride
- aluminum sulfide
- strontium bromide
Answers
-
-
-
- beryllium ion, 2+; oxide ion, 2-
- silver ion, 1+; selenide ion, 2-
- barium ion, 2+; sulfide ion, 2-
- zinc ion, 2+; phosphide ion, 3-
- cesium ion, 1+; fluoride ion, 1-
-
- K2O
- AgI
- Ag2S
- Ba3N2
- CsI
-
- MgCl2
- ZnO
- KF
- Al2S3
- SrBr2





