This is "Unit 3", section 3.1 from the book General Chemistry (v. 1.0).
3.1 Density
Learning Objectives
- To know the definition of density.
- To be able to use the density as a conversion factor to calculate mass or volume.
- To know the definition of specific gravity.
Recall, chemistry is the study of matterAnything that occupies space and has mass., which is anything that has massA fundamental property that does not depend on an object’s location; it is the quantity of matter an object contains. and volumeThe amount of space occupied by an object.. The ratio between the amount of mass and the amount of volume is called the density (d)An intensive property of matter, density is the mass per unit volume (usually expressed in g/cm3). At a given temperature, the density of a substance is a constant.. We will see how to use the density as a conversion factor.
Density is defined as mass per unit volume and is usually expressed in grams per cubic centimeter (g/cm3). As mass increases in a given volume, density also increases. For example, lead, with its greater mass, has a far greater density than the same volume of air, just as a brick has a greater density than the same volume of Styrofoam. At a given temperature and pressure, the density of a pure substance is a constant:
Pure water, for example, has a density of 0.998 g/cm3 at 25°C.
The average densities of some common substances are in Table 3.1(1) "Densities of Common Substances". Notice that corn oil has a lower mass to volume ratio than water. This means that when added to water, corn oil will float. Example 3.1-1 shows how density measurements can be used to identify pure substances.
Table 3.1(1) Densities of Common Substances
| Substance | Density at 25°C (g/cm3) |
|---|---|
| blood | 1.035 |
| body fat | 0.918 |
| whole milk | 1.030 |
| corn oil | 0.922 |
| mayonnaise | 0.910 |
| honey | 1.420 |
Example 3.1-1
The densities of some common liquids are in Table 3.1(2) "Densities of Liquids in Example 3.1-1". Imagine you have five bottles containing colorless liquids (labeled A through E). You must identify them by measuring the density of each. Using a pipette, a laboratory instrument for accurately measuring and transferring liquids, you carefully measure 25.00 mL of each liquid into five beakers of known mass (1 mL = 1 cm3). You then weigh each sample on a laboratory balance. Use the tabulated data to calculate the density of each sample. Based solely on your results, can you unambiguously identify all five liquids?
Masses of samples: A, 17.72 g; B, 19.75 g; C, 24.91 g; D, 19.65 g; E, 27.80 g
Table 3.1(2) Densities of Liquids in Example 3.1-1
| Substance | Density at 25°C (g/cm3) |
|---|---|
| water | 0.998 |
| ethanol (the alcohol in beverages) | 0.789 |
| methanol (wood alcohol) | 0.792 |
| ethylene glycol (used in antifreeze) | 1.113 |
| diethyl ether ("ether"; once widely used as an anesthetic) | 0.708 |
| isopropanol (rubbing alcohol) | 0.785 |
Given: volume and mass
Asked for: density
Strategy:
Step 1 Calculate the density of each liquid from the volumes and masses given.
Step 2 Check to make sure that your answer makes sense.
Step 3 Compare each calculated density with those given in Table 3.1(2) "Densities of Liquids in Example 3.1-1". If the calculated density of a liquid is not significantly different from that of one of the liquids given in the table, then the unknown liquid is most likely the corresponding liquid.
Step 4 If none of the reported densities corresponds to the calculated density, then the liquid cannot be unambiguously identified.
Solution:
Step 1 Density is mass per unit volume and is usually reported in grams per cubic centimeter (or grams per milliliter because 1 mL = 1 cm3). The masses of the samples are given in grams, and the volume of all the samples is 25.00 mL (= 25.00 cm3). The density of each sample is calculated by dividing the mass by its volume. The density of sample A, for example, is
Both the volume and the mass are given to four significant figures, so four significant figures are permitted in the result. The densities of the other samples (in grams per cubic centimeter) are as follows: B, 0.7900; C, 0.9964; D, 0.7860; and E, 1.112.
Step 2 Except for sample E, the calculated densities are slightly less than 1 g/cm3. This makes sense because the masses (in grams) of samples A through D are all slightly less than the volume of the samples, 25.00 mL. In contrast, the mass of sample E is slightly greater than 25 g, so its density must be somewhat greater than 1 g/cm3.
Step 3 Comparing these results with the data given in Table 3.1(2) "Densities of Liquids in Example 3.1-1" shows that sample A is probably diethyl ether (0.708 g/cm3 and 0.7088 g/cm3 are not substantially different), sample C is probably water (0.998 g/cm3 in the table versus 0.9964 g/cm3 measured), and sample E is probably ethylene glycol (1.113 g/cm3 in the table versus 1.112 g/cm3 measured).
Step 4 Samples B and D are more difficult to identify for two reasons: (1) Both have similar densities (0.7900 and 0.7860 g/cm3), so they may or may not be chemically identical. (2) Within experimental error, the measured densities of B and D are indistinguishable from the densities of ethanol (0.789 g/cm3), methanol (0.792 g/cm3), and isopropanol (0.785 g/cm3). Thus some property other than density must be used to identify each sample.
Exercise
Given the volumes and masses of five samples of compounds used in blending gasoline, together with the densities of several chemically pure liquids, identify as many of the samples as possible.
| Sample | Volume (mL) | Mass (g) |
|---|---|---|
| A | 337 | 250.0 |
| B | 972 | 678.1 |
| C | 243 | 190.9 |
| D | 119 | 103.2 |
| E | 499 | 438.7 |
| Substance | Density (g/cm3) |
|---|---|
| benzene | 0.8787 |
| toluene | 0.8669 |
| m-xylene | 0.8684 |
| isooctane | 0.6979 |
| methyl t-butyl ether | 0.7405 |
| t-butyl alcohol | 0.7856 |
Answer: A, methyl t-butyl ether; B, isooctane; C, t-butyl alcohol; D, toluene or m-xylene; E, benzene
Table 3.1(3) Densities of Some Common Substances
| Substance | Density (g/mL or g/cm3) |
|---|---|
| water | 1.0 |
| gold | 19.3 |
| mercury | 13.6 |
| air | 0.0012 |
| cork | 0.22 - 0.26 |
| aluminum | 2.7 |
| iron | 7.87 |
Because of how it is defined, density can act as a conversion factor for switching between units of mass and volume. For example, suppose you have a sample of aluminum that has a volume of 7.88 cm3. How can you determine what mass of aluminum you have without measuring it? You can use the volume to calculate it. If you multiply the given volume by the known density (from Table 3.1(3) "Densities of Some Common Substances"), the volume units will cancel and leave you with mass units, telling you the mass of the sample:
where we have limited our answer to two significant figures.
Example 3.1-2
What is the mass of 44.6 mL of mercury?
Solution
Use the density from Table 3.1(3) "Densities of Some Common Substances" as a conversion factor to go from volume to mass:
Exercise
What is the mass of 25.0 cm3 of iron?
Answer
197 g
Density can also be used as a conversion factor to convert mass to volume; but, care must be taken. We have already demonstrated that the number that goes with density normally goes in the numerator when density is written as a fraction. Take the density of gold, for example:
Although this was not previously pointed out, it can be assumed that there is a 1 in the denominator:
That is, the density value tells us that we have 19.3 grams for every 1 milliliter of volume, and the 1 is an exact number. When we want to use density to convert from mass to volume, the numerator and denominator of density need to be switched. This way the mass unit will cancel and the volume unit will be introduced. Thus, if we want to know the volume of 45.9 g of gold, we would set up the conversion as follows:
Note how the mass units cancel, leaving the volume unit.
Example 3.1-3
A cork stopper from a bottle of wine has a mass of 3.78 g. If the density of cork is 0.22 g/cm3, what is the volume of the cork?
Solution
We arrange the numerator and denominator of the density as a conversion factor that cancels mass and gives us volume. So
Exercise
What is the volume of 3.78 g of gold?
Answer
0.196 cm3
Care must be used with density as a conversion factor. Make sure the mass units are the same, or the volume units are the same, before using density to convert to a different unit. Often, the unit of the given quantity must be first converted to the appropriate unit before applying density as a conversion factor.
Specific Gravity
Specific gravity is defined as the density of a substance divided by the density of water. As such, specific gravity is a dimensionless number that gives an immediate comparison to the density of water. A specific gravity greater than 1 indicates the density of the substance is greater than the density of the water. Since the density of water, though temperature dependent, is often taken to be 1 g/mL, the specific gravity can in most cases be considered simply the numerical value of the density, but without units. To measure the specific gravity, one employs a device called a hydrometer.
Figure 3.1(a)Schematic of a Hydrometer
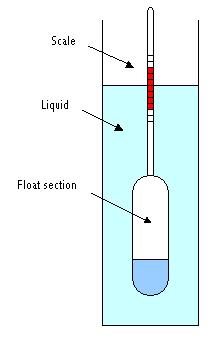
Image Credit: By P.wormer (Own work) [CC BY-SA 3.0 (http://creativecommons.org/licenses/by-sa/3.0) or GFDL (http://www.gnu.org/copyleft/fdl.html)], via Wikimedia Commons
Figure 3.1(b)Hydrometer in Beer

Hydrometer floating in a test jar of beer wort. The specific gravity reading is approximately 1.050 Image Credit: By Schlemazl (Own work) [CC BY-SA 3.0 (http://creativecommons.org/licenses/by-sa/3.0)], via Wikimedia Commons
Summary
Density relates how much matter is packed into an amount of space. We can use density conceptually to decide if an object will float in something else. We can also use density as a conversion factor between mass and volume. Specific gravity is the density of a substance divided by the density of water and is dimensionless.





