This is "Unit 2", section 2.3 from the book General Chemistry (v. 1.0).
2.3 Other Applications of Conversion Factors
Learning Objective
- To be able to convert area and volume units within and between the metric and English systems.
- To be able to perform calculations involving rates such as meters per second, or miles per gallon.
- To be able to convert measurements expressed as percentages.
By now you should be able to do any conversion of length or mass within and between the metric and/or English systems of measurement. You hopefully recognize every factor you use is simply the number 1 expressed as a fraction where the numerator and denominator have units. Since the numerator and denominator are equal, as long as they have units, using conversion factors is always just multiplication by the number 1. In this section we will ratchet up the complexity of the problems; however, it is critical to recognize we are still simply multiplying by 1.
Volumes and Areas
Volumes are often expressed with units of cubic lengths, for example, cubic inches (in3), or cubic meters (m3). Areas are expressed with units of square lengths, for example houses are often valued by square feet (ft2). One has to be careful converting volumes and areas such as these.
Figure 2.3(a)1 m3 of Potatoes and Barbies

Gerhard Vormwald [GFDL (http://www.gnu.org/copyleft/fdl.html) or CC BY-SA 4.0-3.0-2.5-2.0-1.0 (http://creativecommons.org/licenses/by-sa/4.0-3.0-2.5-2.0-1.0)], via Wikimedia Commons
From Table 1.4(2) we know that 1 m = 100 cm. But beware you don't make the rookie mistake and say 1 m3 = 100 cm3. As figure 2.3(a) shows, 1 m3= 100 cm × 100 cm × 100 cm = 106 cm3.
Areas can be expressed in terms of square inches (in2). The tile pictured in figure 2.3(b) would have an area of 60 in2 (rounded to one significant figure).
Figure 2.3(b)Eight-inch square tile painted by Winslow Homer, 1878
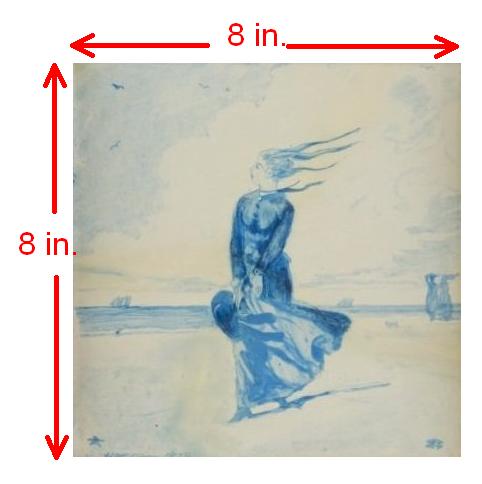
Winslow Homer [Public domain], via Wikimedia Commons
Converting the 60 in2 into square cm (cm2) could involve first converting the 8 in into cm.
(8 in.)= 20.32 cm
Then, the result would need to be squared to make it the area of the tile.
(20.32 cm)2 = 412.9 cm2 = 400 cm2 (rounded to one sig. fig).
Rates
We are often presented with factors relating two distinct variables which we can think of as rates. For example, a car might have a gas mileage rate of 35 miles per gallon (mpg). If we wanted to know how many miles we could drive that car on say 9.0 gallons of gas, we could use the gas mileage as a conversion factor:
(9.0 gal)= 320 miles
Alternatively, if we wanted to know how many gallons of gas it would take to drive 74 miles, we could again use the gas mileage as a conversion factor:
(74 miles)= 2.1 gallons
We can extend this discussion to money. A price is just another type of rate and is therefore a conversion factor. For example, if we wanted to know how much it cost (in gas money) to drive 152 miles if our car got 32 mpg and gas cost $3.29 per gallon.
(152 miles)= $16
Percentages
Figure 2.3(c)Zinc Sulfide in a Weighing Bottle

By Skdmov (Own work) [CC BY-SA 4.0 (http://creativecommons.org/licenses/by-sa/4.0)], via Wikimedia Commons
Percentages are a special type of rate that indicate a portion's share of a total based upon 100. In considering the elemental composition of pure substances chemists often use percentages by mass. For example, a chemist might know that a 22.43 g-sample of zinc sulfide (ZnS) was 67.09% zinc. A chemist could then calculate the number of grams of zinc were in the sample this way:
(22.43 g sample)= 15.05 g zinc
Alternatively, a chemist might know she needed 1.63 g of zinc for a reaction from a sample of zinc sulfide that was 67.09% zinc by mass. She could calculate the number of grams of zinc sulfide she needed as follows:
(1.63 g zinc)= 2.43 g zinc sulfide
Summary
Conversion factors have a huge number of applications including volumes, rates and percentages. One first identifies the relationship between the variables and then arranges a factor with units such that the units cancel as desired.
Example 2.3-1
Courtesy of Fred Redmore, Highland Community College
-
Perform the following conversions:
- If a car is traveling at a rate of 100 km/hr, how many meters will it travel in 1.0 minute?
- If a person walks at a rate of 3.5 mi/hr, how many minutes will it take to walk 5.8 km? (1 km = 0.62 mi)
- If a car is traveling at a rate of 55 mi/hr, how many cm will it travel in 1.0 minute? (1 km = 0.62 mi)
- If you are riding a bicycle at a rate of 15 km/hr, how many minutes will it take to travel 6.25 m?
-
How many grams of silver are in 27.4 g of a compound that is 63.5% silver?
The engine pictured below has a cylinder displacement of 402 in3. What is the displacement in L?

A Roush 402 cubic inch engine built for the Shelby Daytona Coupe. Image Credit: By Sodfijd at English Wikipedia [Public domain], via Wikimedia Commons
Answers
-
(1 min.)= 1.7 × 103 m
- (5.8 km)= 62 minutes
- (1 min)= 1.5 × 105 cm
- (6.25 m)= 0.025 min
-
(27.4 g compound)= 17.4 g silver





