This is "Unit 3", section 3.2 from the book General Chemistry (v. 1.0).
3.2 Temperature
Learning Objectives
- To know the definition of absolute temperature.
- To distinguish absolute temperature scales from other temperature systems.
- To be able to derive equations that convert temperatures in kelvin, Celsius or Fahrenheit to temperatures in any other system.
Chapter 2 introduced dimensional analysis, a technique you need to successfully manipulate mathematical equations in chemistry. This section describes how to convert between temperature scales and further develops the topic of unit conversions started in previous sections.
Temperature
The concept of temperature may seem familiar to you, but many people confuse temperature with heat. Temperature is a measure of how hot or cold an object is relative to another object (its thermal energy content), whereas heat is the flow of thermal energy between objects with different temperatures.
Three different scales are commonly used to measure temperature: Fahrenheit (expressed as °F), Celsius (°C), and Kelvin (K). Thermometers measure temperature by using materials that expand or contract when heated or cooled. Mercury or alcohol thermometers, for example, have a reservoir of liquid that expands when heated and contracts when cooled, so the liquid column lengthens or shortens as the temperature of the liquid changes.
The Fahrenheit Scale
The Fahrenheit temperature scale was developed in 1717 by the German physicist Gabriel Fahrenheit, who designated the temperature of a bath of ice melting in a solution of salt as the zero point on his scale. Such a solution was commonly used in the 18th century to carry out low-temperature reactions in the laboratory. The scale was measured in increments of 12; its upper end, designated as 96°, was based on the armpit temperature of a healthy person—in this case, Fahrenheit’s wife. Later, the number of increments shown on a thermometer increased as measurements became more precise. The upper point is based on the boiling point of water, designated as 212° to maintain the original magnitude of a Fahrenheit degree, whereas the melting point of ice is designated as 32°.
The Celsius Scale
The Celsius scale was developed in 1742 by the Swedish astronomer Anders Celsius. It is based on the melting and boiling points of water under normal atmospheric conditions. The current scale is an inverted form of the original scale, which was divided into 100 increments. Because of these 100 divisions, the Celsius scale is also called the centigrade scale.
The Kelvin Scale
Lord Kelvin, working in Scotland, developed the Kelvin scale in 1848. His scale uses molecular energy to define the extremes of hot and cold. Absolute zero, or 0 K, corresponds to the point at which molecular energy is at a minimum. The Kelvin scale is preferred in scientific work since the temperature in kelvin is proportional to the average kinetic energy of the molecules in the sample. The Celsius scale is also commonly used, mostly because so much lab work happens in the neighborhood of room temperature to boiling water temperature (25 °C to 100 °C). Temperatures measured on the Kelvin scale are reported simply as K, not °K. Figure 5.25 "A Comparison of the Fahrenheit, Celsius, and Kelvin Temperature Scales" compares the three scales.
Figure 3.2(a) A Comparison of the Fahrenheit, Celsius, and Kelvin Temperature Scales
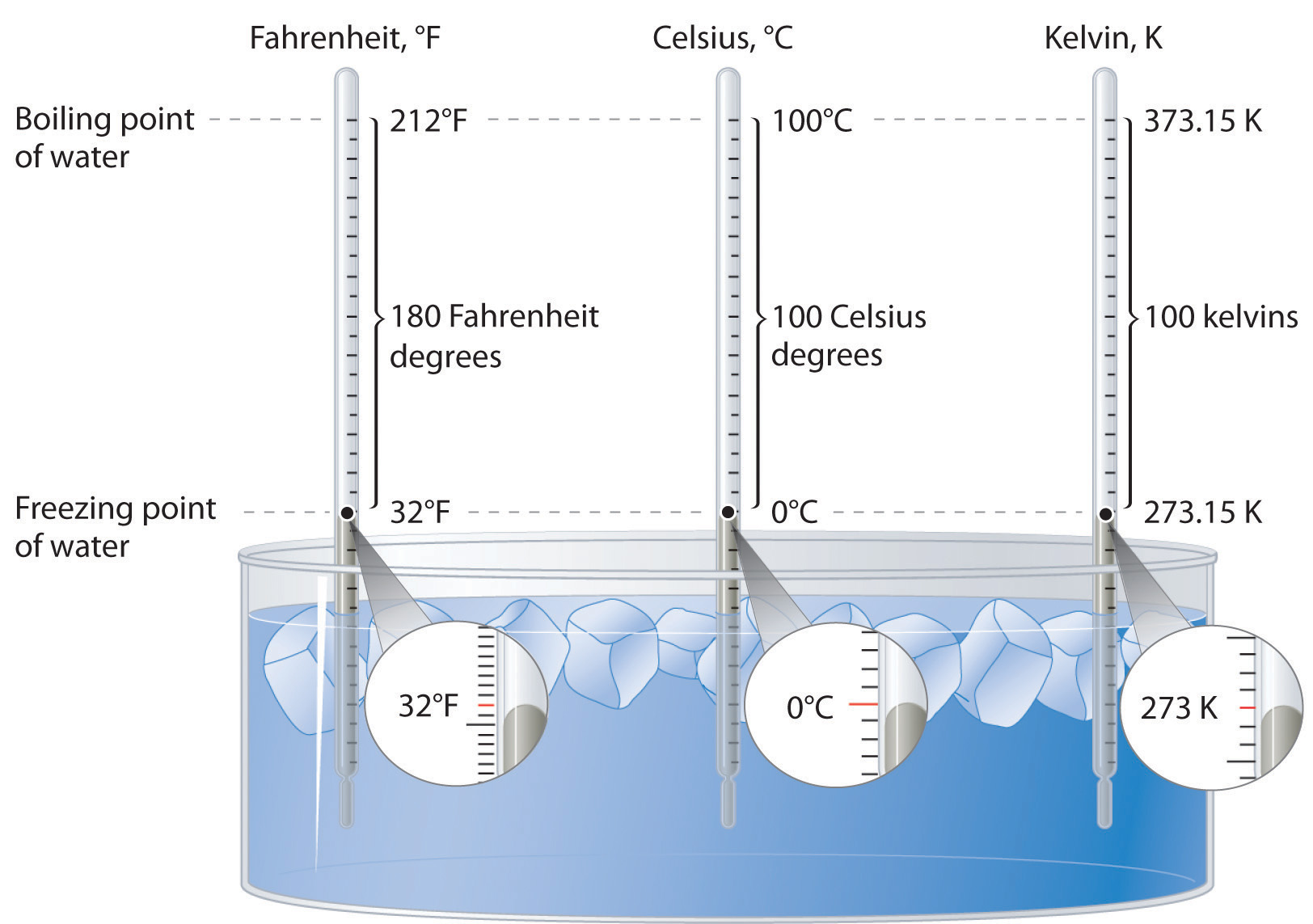
Because the difference between the freezing point of water and the boiling point of water is 100° on both the Celsius and Kelvin scales, the size of a degree Celsius (°C) and a kelvin (K) are precisely the same. In contrast, both a degree Celsius and a kelvin are 9/5 the size of a degree Fahrenheit (°F).
Converting between Scales
The kelvin is the same size as the Celsius degree, so measurements are easily converted from one to the other. The freezing point of water is 0°C = 273.15 K (not a number you need to memorize); the boiling point of water is 100°C = 373.15 K. The Kelvin and Celsius scales are related as follows:
T (in °C) + 273.15 = T (in K) T (in K) − 273.15 = T (in °C)To convert temperatures to or from the Fahrenheit scale is more complex than the simple addition or subtraction operation needed for Celsius/Kelvin conversions. Not only do you need to consider the different zero points, you also need to consider the size of the degree being different. Start by considering figure 3.2(a). Construct a graph of the data presented by putting Celsius on the y-axis and Fahrenheit on the x-axis.
Figure 3.2(b) Graph of Data from Figure 3.2(a)
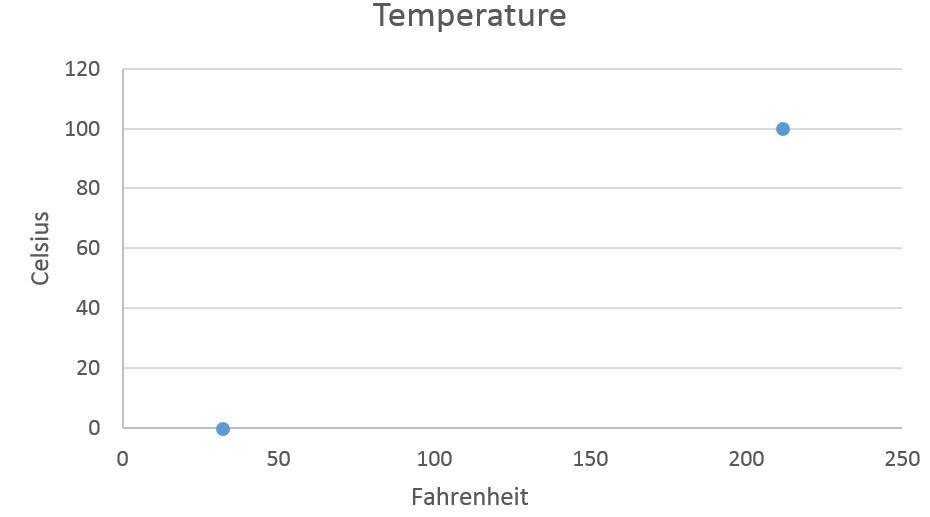
Now write an equation of the line that connects the two points. You know the slope, .
So in y = mx + b form,
To figure out a value for b, simple plug in any point on the graph. It would be simplest to choose the point (0,32). Yielding the following equation
Solve for b,
b = -17.778 °C
And now you have an equation that converts any temperature in °C to °F.
It's a matter of algebra, left to the reader, to rearrange the equation to solve for °F given °C. The resulting equation, however, is given in Example 3.2-1 (below).
There is only one temperature for which the numerical value is the same on both the Fahrenheit and Celsius scales: −40°C = −40°F.
Example 3.2-1
- Convert the temperature of the surface of the sun (5800 K) and the boiling points of gold (3080 K) and liquid nitrogen (77.36 K) to °C and °F.
- A student is ill with a temperature of 103.5°F. What is her temperature in °C and K?
Solution:





