This is "Unit 5", section 5.2 from the book General Chemistry (v. 1.0).
5.2 Acids and Bases
Learning Objective
- To identify and name some common acids and bases.
For our purposes at this point in the text, we can define an acidA substance with at least one removable hydrogen atom. as a substance with at least one removable hydrogen ion. We can define basesA substance that can remove a hydrogen ion from an acid. as compounds that can remove a hydrogen ion from an acid. Solutions that are neither basic nor acidic are neutral. We will discuss the chemistry of acids and bases in more detail in future chapters, but in this section we describe the nomenclature of common acids and identify some important bases so that you can recognize them in future discussions. Pure acids and bases and their concentrated aqueous solutions are commonly encountered in the laboratory. They are usually highly corrosive, so they must be handled with care.
Acids
The names of acids differentiate between (1) acids in which the H+ ion is attached to an oxygen atom (these are called oxoacidsAn acid in which the dissociable ion is attached to an oxygen atom of a polyatomic anion., or occasionally oxyacids) and (2) acids in which the H+ ion is attached to some other element besides oxygen. In the latter case, the name of the acid begins with hydro- and ends in -ic, with the root of the name of the other element or ion in between. Recall that the name of the anion derived from this kind of acid always ends in -ide. Thus hydrogen chloride (HCl) gas dissolves in water to form hydrochloric acid (which contains H+ and Cl− ions), hydrogen cyanide (HCN) gas forms hydrocyanic acid (which contains H+ and CN− ions), and so forth (Table 5.2(1) "Some Common Acids That Do Not Contain Oxygen"). Examples of this kind of acid are commonly encountered and very important. For instance, your stomach contains a dilute solution of hydrochloric acid to help digest food. When the mechanisms that prevent the stomach from digesting itself malfunction, the acid destroys the lining of the stomach and an ulcer forms.
Note the Pattern
Acids are distinguished by whether the H+ ion is attached to an oxygen atom of a polyatomic anion or some other element.
Table 5.2(1) Some Common Acids That Do Not Contain Oxygen
| Formula | Name in Aqueous Solution | Name of Gaseous Species |
|---|---|---|
| HF | hydrofluoric acid | hydrogen fluoride |
| HCl | hydrochloric acid | hydrogen chloride |
| HBr | hydrobromic acid | hydrogen bromide |
| HI | hydroiodic acid | hydrogen iodide |
| HCN | hydrocyanic acid | hydrogen cyanide |
| H2S | hydrosulfuric acid | hydrogen sulfide |
If an acid contains one or more H+ ions attached to oxygen, a "hydro-" prefix is not permitted in the name. Instead the names of the oxoacids usually start with the beginning of the name of the other element connected to the oxygen. Sometimes, as shown in figure 5.2(a), a prefix of per- or hypo is applied when there are several possibilities for the number of oxygens. The names of the oxyacids end in -ic or -ate as shown inf Figure 5.2(a) and Table 5.2(2). By the way, at this point it is critical for you to memorize the names and formulas of the acids in Tables 5.2(1), Table 5.2(2) and Figure 5.2(a).
Figure 5.2(a) The Relationship between the Names of the Oxoacids and the Names of the Parent Oxoanions
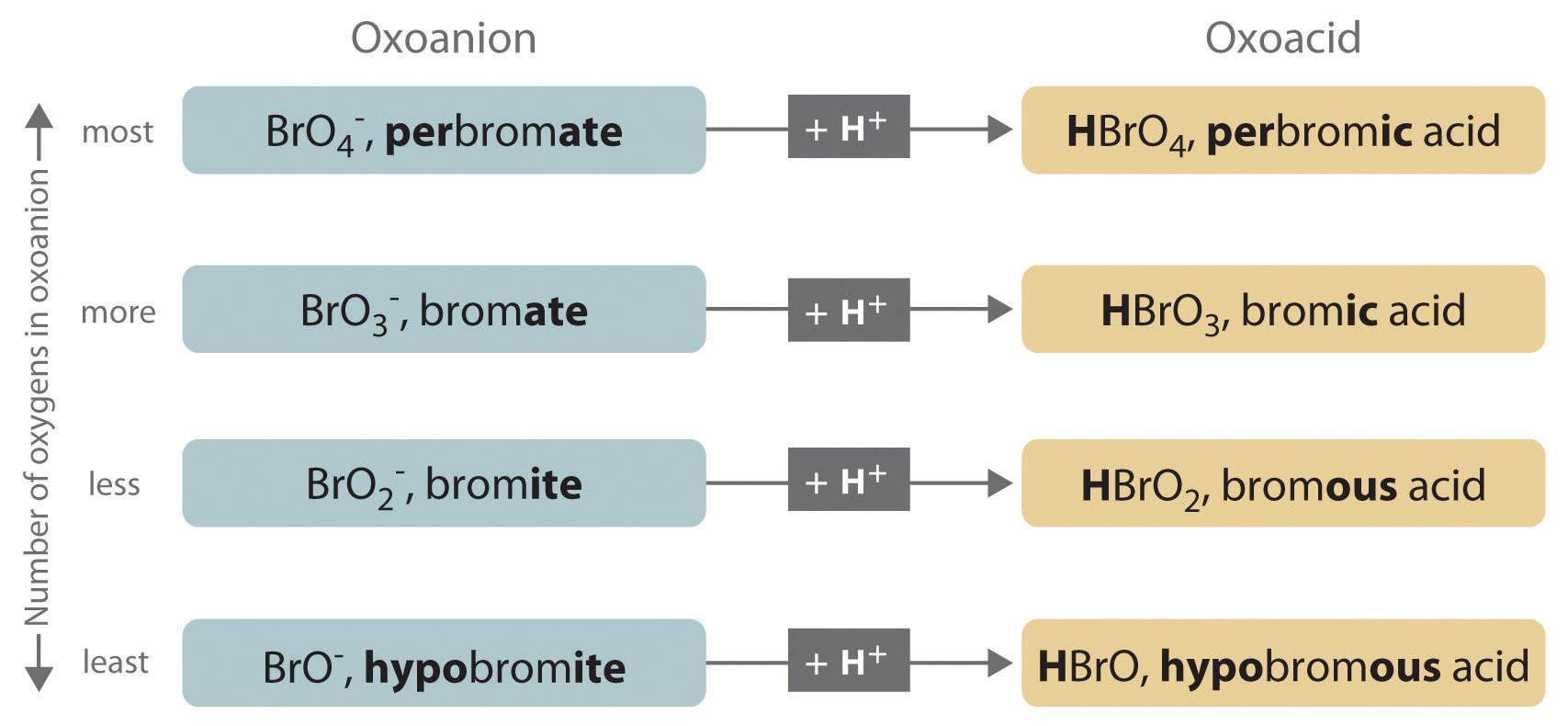
Table 5.2(2) Some Common Oxoacids
| Formula | Name |
|---|---|
| HNO2 | nitrous acid |
| HNO3 | nitric acid |
| H2SO3 | sulfurous acid |
| H2SO4 | sulfuric acid |
| H3PO4 | phosphoric acid |
| HC2H3O2 | acetic acid |
| H2CO3 | carbonic acid |
| HClO | hypochlorous acid |
| HClO2 | chlorous acid |
| HClO3 | chloric acid |
| HClO4 | perchloric acid |
Bases
Many compounds can act as bases. With one important exception, virtually every base you encounter will be an ionic compound. For example, ionic compounds such as sodium hydroxide (NaOH) and barium hydroxide [Ba(OH)2], that contain the hydroxide ion and a metal cation. These have the general formula M(OH)n. Salts derived from the oxyoacids are also basic. For example, sodium phosphate derived from phosphoric acid and potassium carbonate are important bases.
Now for the exceptions alluded to in the previous paragraph, the basic compounds that are not ionic. Ammonia, NH3, is a molecular compound commonly used as a base. Replacing a hydrogen atom of NH3 with an carbon group results in an amineAn organic compound that has the general formula , where R is an carbon group. Amines, like ammonia, are bases. (RNH2), which is also a base. Amines have pungent odors—for example, methylamine (CH3NH2) is one of the compounds responsible for the foul odor associated with spoiled fish. The physiological importance of amines is suggested in the word vitamin, which is derived from the phrase vital amines. The word was coined to describe dietary substances that were effective at preventing scurvy, rickets, and other diseases because these substances were assumed to be amines. Subsequently, some vitamins have indeed been confirmed to be amines.
Note the Pattern
Metal hydroxides (MOH), metal oxides, carbonates, phosphates and ammonia are common bases.
Summary
Common acids have their own names and rules for nomenclature. The nomenclature of acids differentiates between oxoacids, in which the H+ ion is attached to an oxygen atom , and acids in which the H+ ion is attached to another element. Metal hydroxides (MOH) are bases. Ammonia is an important base, as are its organic derivatives, the amines.
Key Takeaway
- Common acids and polyatomic anions derived from them have their own names and rules for nomenclature.
Conceptual Problems
-
Name each acid.
- HCl
- HBrO3
- HNO3
- H2SO4
- HIO3
-
Name each acid.
- HBr
- H2SO3
- HClO3
- HCN
- H3PO4
-
Name the aqueous acid that corresponds to each gaseous species.
- hydrogen bromide
- hydrogen cyanide
- hydrogen iodide
-
When each compound is added to water, is the resulting solution acidic, neutral, or basic?
- CH3CH2OH
- Mg(OH)2
- LiOH
- C3H7CO2H
- H2SO4
Answers
-
-
-
-
- This is an alcohol (R-OH) and so is neutral.
- Metal hydroxides are basic in aqueous solution.
- basic
- This is an organic acid analogous to HC2H3O2.
- acidic
Numerical Problems
-
Write the formula for each compound.
- hypochlorous acid
- perbromic acid
- hydrobromic acid
- sulfurous acid
-
Write the formula for each compound.
- hydroiodic acid
- hydrogen sulfide
- phosphorous acid
- perchloric acid





