This is "Unit 4", section 4.1 from the book General Chemistry (v. 1.0).
4.1 Bonding in Elements
Learning Objective
- To understand the differences between covalent and ionic bonding.
Figure 4.1(a) Forces Between Atoms
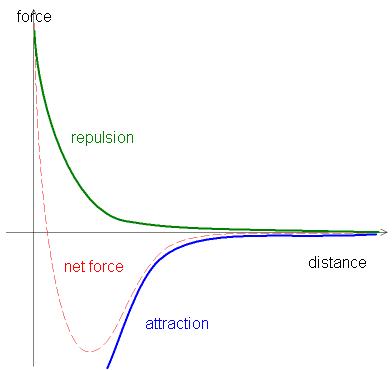
The green line represents the interaction between like charged particles, for example, the electron cloud of one atom interacting with the electron cloud of another atom. The blue line represents the interaction between opposite charges, for example, the nucleus of one atom and the electron cloud of another atom. The red line represents the sum of the forces present between two atoms. By Christophe Dang Ngoc Chan [CC BY-SA 3.0] via Wikimedia Commons
Until now in this course we have limited our discussion to isolated atoms. When you bring atoms a certain distance apart, there is a tendency for them to hook up. We call the hook up a chemical bond. The atoms in all substances that contain more than one atom are held together by electrostatic interactionsAn interaction between electrically charged particles such as protons and electrons.--interactions between electrically charged particles such as protons and electrons. Electrostatic attractionAn electrostatic interaction between oppositely charged species (positive and negative) that results in a force that causes them to move toward each other. between oppositely charged species (positive and negative) results in a force that causes them to move toward each other, like the attraction between opposite poles of two magnets. In contrast, electrostatic repulsionAn electrostatic interaction between two species that have the same charge (both positive or both negative) that results in a force that causes them to repel each other. between two species with the same charge (either both positive or both negative) results in a force that causes them to repel each other, as do the same poles of two magnets. Atoms form chemical compounds when the attractive electrostatic interactions between them are stronger than the repulsive interactions. Collectively, we refer to the attractive interactions between atoms as chemical bondsAn attractive interaction between atoms that holds them together in compounds..
Covalent Bonds in Elements
One type of chemical bond is called a covalent bond. A covalent bond is characterized by a shared pair of electrons. A multi-atom entity joined by covalent bonds is called a molecule. Some elements, rather than existing as discrete atoms, instead exist as molecules. Hydrogen, nitrogen, oxygen, and the halogens occur naturally as the diatomic ("two atoms") molecules H2, N2, O2, F2, Cl2, Br2, and I2 (part (a) in Figure 4.1(b)"). Similarly, a few other elements are polyatomicMolecules that contain more than two atoms. ("many atoms") molecules, such as elemental phosphorus and sulfur, which occur as P4 and S8 (part (b) in Figure 4.1(b).)
Each covalent compound is represented by a molecular formula.A representation of a covalent compound that consists of the atomic symbol for each component element (in a prescribed order) accompanied by a subscript indicating the number of atoms of that element in the molecule. , which gives the atomic symbol for each component element, in a prescribed order, accompanied by a subscript indicating the number of atoms of that element in the molecule. The subscript is written only if the number of atoms is greater than 1.
Figure 4.1(b) parts (a) and (b) Elements That Exist as Covalent Molecules
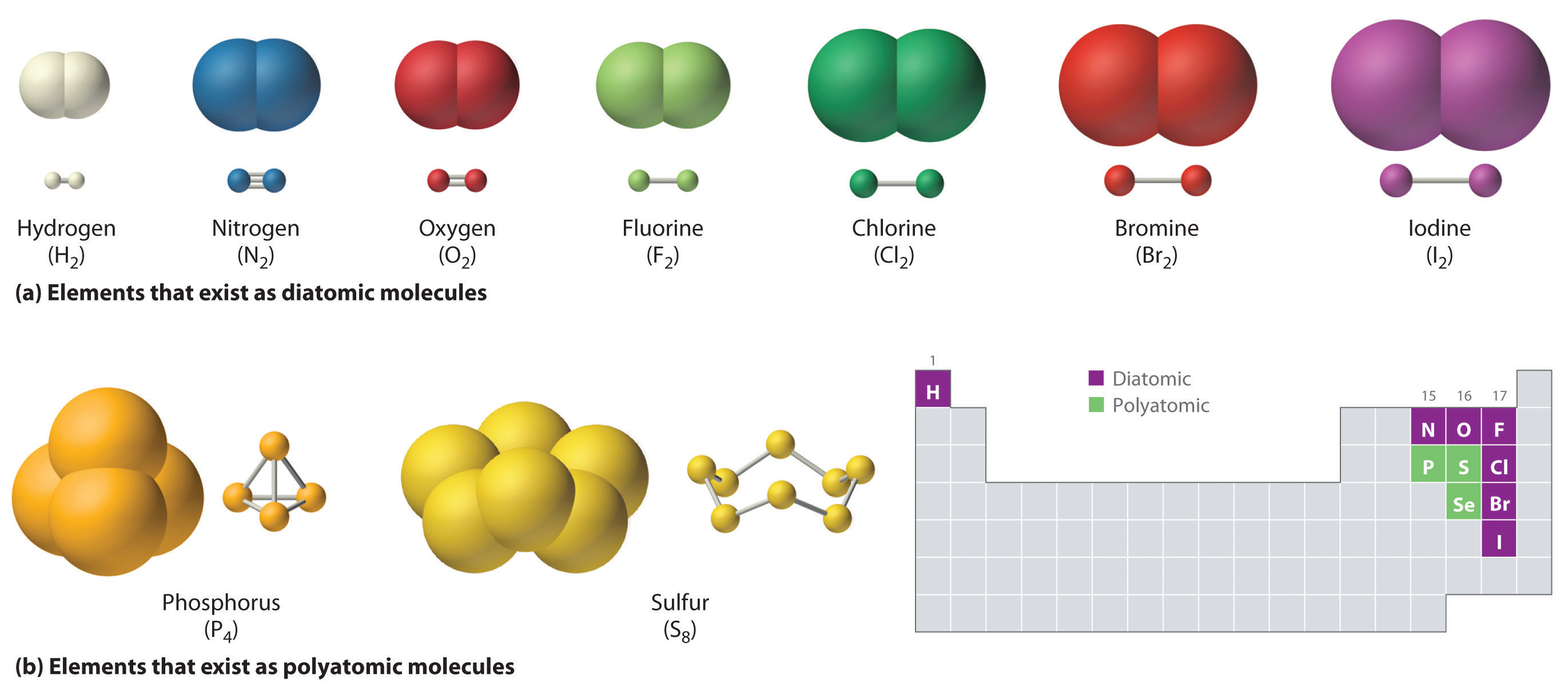
(a) Several elements naturally exist as diatomic molecules, in which two atoms (E) are joined by one or more covalent bonds to form a molecule with the general formula E2. (b) A few elements naturally exist as polyatomic molecules, which contain more than two atoms. For example, phosphorus exists as P4 tetrahedra--regular polyhedra with four triangular sides--with a phosphorus atom at each vertex. Elemental sulfur consists of a puckered ring of eight sulfur atoms connected by single bonds. Selenium is not shown due to the complexity of its structure.
Representations of Molecular Structures
Molecular formulas give only the elemental composition of molecules. In contrast, structural formulasA representation of a molecule that shows which atoms are bonded to one another and, in some cases, the approximate arrangement of atoms in space. show which atoms are bonded to one another and, in some cases, the approximate arrangement of the atoms in space. Knowing the structural formula of a compound enables chemists to create a three-dimensional model, which provides information about how that compound will behave physically and chemically.
The structural formula for H2 can be drawn as H-H and that for I2 as I-I, where the line indicates a single pair of shared electrons, a single bondA chemical bond formed when two atoms share a single pair of electrons.. Two pairs of electrons are shared in a double bondA chemical bond formed when two atoms share two pairs of electrons., which is indicated by two lines, for example, O2 is O=O. Three electron pairs are shared in a triple bondA chemical bond formed when two atoms share three pairs of electrons., which is indicated by three lines, for example, N2 is N≡N (see Figure 4.1(c)). We will be seeing how to figure out the bonding arrangements in chapter 6. For now just remember hydrogen, oxygen, nitrogen, and carbon have a very strong tendency to form substances in which they have one, two, three, and four bonds to other atoms, respectively (Table 4.1(1) "The Number of Bonds That Selected Atoms Commonly Form to Other Atoms").
Figure 4.1(c) Molecules That Contain Single, Double, and Triple Bonds
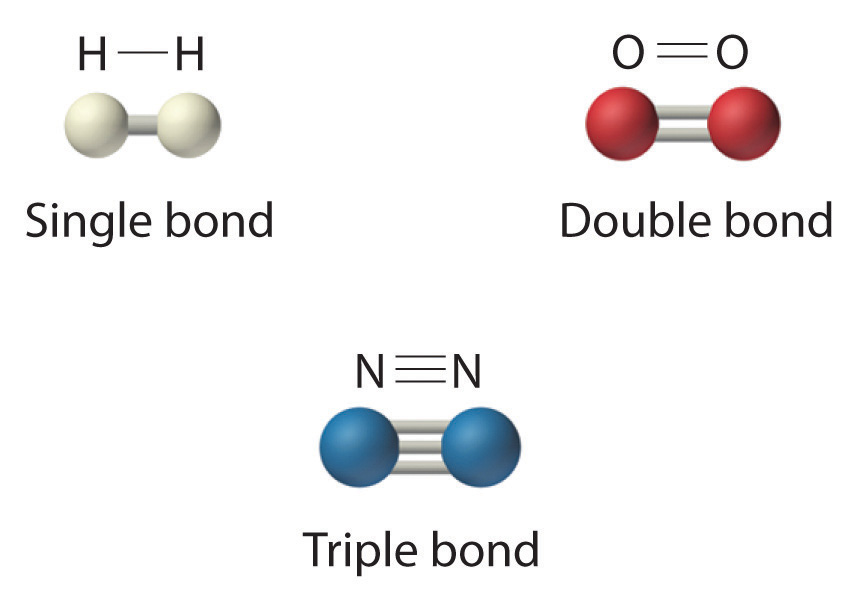
Hydrogen (H2) has a single bond between atoms. Oxygen (O2) has a double bond between atoms, indicated by two lines (=). Nitrogen (N2) has a triple bond between atoms, indicated by three lines (≡). Each bond represents an electron pair.
Table 4.1(1) The Number of Bonds That Selected Atoms Commonly Form to Other Atoms
| Atom | Number of Bonds |
|---|---|
| H (group 1) | 1 |
| O (group 16) | 2 |
| N (group 15) | 3 |
| C (group 14) | 4 |
Summary
The atoms in chemical compounds are held together by attractive electrostatic interactions known as chemical bonds. Some elements consist of molecules, groups of atoms in which one or more pairs of electrons are shared by at least two atoms to form a covalent bond. The atoms in molecules are held together by the electrostatic attraction between the positively charged nuclei of the bonded atoms and the negatively charged electrons shared by the nuclei. The molecular formula of a covalent compound gives the types and numbers of atoms present. Diatomic molecules contain two atoms, and polyatomic molecules contain more than two. A structural formula indicates the composition and approximate structure and shape of a molecule. Single bonds, double bonds, and triple bonds are covalent bonds in which one, two, and three pairs of electrons, respectively, are shared between two bonded atoms.





