This is "Unit 8", section 8.2 from the book General Chemistry (v. 1.0).
8.2 Assigning Oxidation Numbers
Learning Objectives
- To identify fundamental types of chemical reactions.
- To predict the types of reactions substances will undergo.
In section 7.2 we defined oxidation as any chemical change in which a species LOSES electrons. Conversely, reduction is any chemical change in which a species gains electrons. These definitions beg the question "How do you know where the electrons came from or where the electrons went?" Chemists have devised a bookeeping system for electrons that lets us keep track of the flow of electrons in a chemical change simply by considering the formulas of the reactants and products. This bookeeping system depends on the assignment of an oxidation state (or oxidation number). This page gives the rules for using the oxidation number system and a little practice.
Assigning Oxidation States
So far, with respect to our three classifications of chemical change, you had to memorize the solubility rules for the precipitation reactions. For the acid-base neutralization reactions, you had to memorize which substances are acids and which substances are bases. For oxidation-reduction reactions, you must memorize some rules for our electron-bookeeping system.
Rules for Assigning Oxidation States
- The oxidation state of an atom in any pure element, whether monatomic, diatomic, or polyatomic, is zero.
- The oxidation state of a monatomic ion is the same as its charge—for example, Na+ = +1, Cl− = −1.
- The oxidation state of fluorine in chemical compounds is always −1. Other halogens usually have oxidation states of −1 as well, except when combined with oxygen or other halogens.
- Hydrogen is assigned an oxidation state of +1 in its compounds with nonmetals and −1 in its compounds with metals.
- Oxygen is normally assigned an oxidation state of −2 in compounds, with two exceptions: in compounds that contain oxygen–fluorine or oxygen–oxygen bonds, the oxidation state of oxygen is determined by the oxidation states of the other elements present.
- The sum of the oxidation states of all the atoms in a neutral molecule or ion must equal the charge on the molecule or ion.
A frequently made rookie mistake is to learn just the first two rules and then make the leap that oxidation state is the same as electric charge. Oxidation state and charge are NOT the same. Remember that oxidation states are useful for visualizing the transfer of electrons in oxidation–reduction reactions, but the oxidation state of an atom and its actual charge are the same only for simple ionic compounds. Oxidation states are a convenient way of assigning electrons to atoms, and they are useful for predicting the types of reactions that substances undergo. An electric charge is a real quantity that can be measured in the lab and assigned units. An oxidation state cannot be measured, it is merely an intellectual contrivance.
Example 8.2-1
Assign oxidation states to all atoms in each compound.
- sulfur hexafluoride (SF6)
- methanol (CH3OH)
- ammonium sulfate [(NH4)2SO4]
- nitrogen dioxide (NO2)
- dinitrogen monoxide (N2O)
Given: molecular or empirical formula
Asked for: oxidation states
Strategy:
Begin with atoms whose oxidation states can be determined unambiguously from the rules presented (such as fluorine, other halogens, oxygen, and monatomic ions). Then determine the oxidation states of other atoms present according to rule 1.
Solution:
We know from rule 3 that fluorine always has an oxidation state of −1 in its compounds. Each of the six fluorine atoms in sulfur hexafluoride count −1. This give a total of −6 from fluorine. Because rule 1 requires that the sum of the oxidation states of all atoms be zero in a neutral molecule (here SF6), the oxidation state of sulfur must be +6:
[(6 F atoms)(−1)] + [(1 S atom) (+6)] = 0According to rules 4 and 5, hydrogen and oxygen have oxidation states of +1 and −2, respectively. Because methanol has no net charge, carbon must have an oxidation state of −2:
[(4 H atoms)(+1)] + [(1 O atom)(−2)] + [(1 C atom)(−2)] = 0Note that (NH4)2SO4 is an ionic compound that consists of both a polyatomic cation (NH4+) and a polyatomic anion (SO42−) . We assign oxidation states to the atoms in each polyatomic ion separately. For NH4+, hydrogen has an oxidation state of +1 (rule 4), so nitrogen must have an oxidation state of −3:
[(4 H atoms)(+1)] + [(1 N atom)(−3)] = +1, the charge on the NH4+ ionFor SO42−, oxygen has an oxidation state of −2 (rule 5), so sulfur must have an oxidation state of +6:
[(4 O atoms) (−2)] + [(1 S atom)(+6)] = −2, the charge on the sulfate ionOxygen has an oxidation state of −2 (rule 5), giving an overall contribution to the oxidation number of −4 per formula unit. This must be balanced by the positive contribution on the nitrogen atom, giving an oxidation state of +4 for nitrogen:
Oxygen has an oxidation state of −2 (rule 5), giving an overall contribution to the oxidation number of −2 per formula unit. This must be balanced by the positive contribution on the two nitrogen atoms, giving an oxidation state of +1 for nitrogen:
Exercise
Assign oxidation states to all atoms in each compound.
- barium fluoride (BaF2)
- formaldehyde (CH2O)
- potassium dichromate (K2Cr2O7)
- cesium oxide (Cs2O)
- ethanol (CH3CH2OH)
Answer:
- Ba, +2; F, −1
- C, 0; H, +1; O, −2
- K, +1; Cr, +6; O, −2
- Cs, +1; O, −2
- C(average), -2; H, +1; O, −2
Summary
This section lays a foundation for looking at oxidation-reduction reactions in more depth in the next section. Oxidation is any chemical change in which a substance loses electrons. Reduction is any chemical change in which a substance gains eletrons. To keep track of electrons in chemical reactions, oxidation states are assigned to atoms in compounds.
Key Takeaway
- Memorize the six rules for assigning oxidation state.
Numerical Problems
-
Assign oxidation states to the atoms in each compound or ion.
- (NH4)2S
- the phosphate ion
- [AlF6]3−
- CuS
- HCO3−
- NH4+
- H2SO4
- formic acid, CH2O2
-
Assign oxidation states to the atoms in each compound or ion.
- ClO2
- HO2−
- sodium bicarbonate
- MnO2
- PCl5
- [Mg(H2O)6]2+
- N2O4
- methanol, CH4O
-
Assign oxidation states to the atoms in each compound.
- iron(III) nitrate
- Al2O3
- potassium sulfate
- Cr2O3
- sodium perchlorate
- Cu2S
- hydrazine (N2H4)
- NO2
- n-pentanol
-
Assign oxidation states to the atoms in each compound.
- calcium carbonate
- NaCl
- CO2
- potassium dichromate
- KMnO4
- iron(III) oxide
- Cu(OH)2
- Na2SO4
- n-hexanol
Answers
-
- S, −2; N, −3; H, +1
- P, +5; O, −2
- F, −1; Al, +3
- S, −2; Cu, +2
- H, +1; O, −2; C, +4
- H, +1; N, −3
- H, +1; O, −2; S, +6
- H, +1, O, −2; C, +2
-
-
-
- Ca, +2; O, −2; C, +4
- Na, +1; Cl, −1
- O, −2; C, +4
- K, +1; O, −2; Cr, +6
- K, +1; O, −2; Mn, +7
- O, −2; Fe, +3
- O, −2; H, +1; Cu, +2
- O, −2; S, +6
-
Hexanol
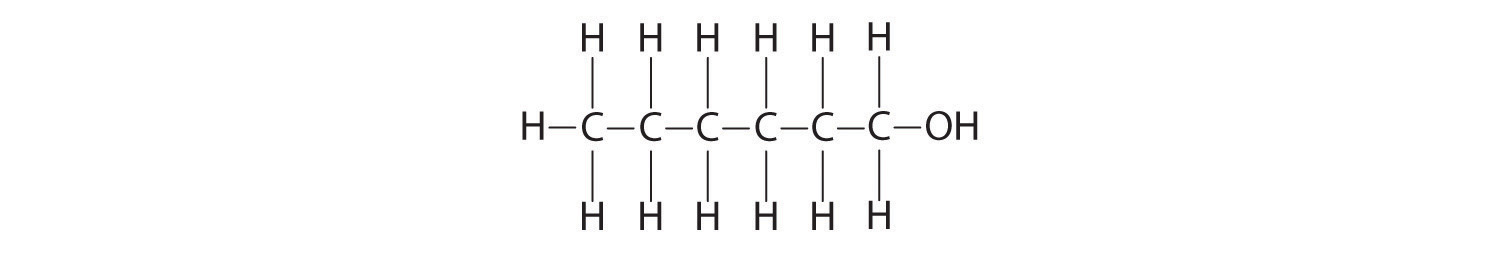
O,−2; H, +1
From left to right: C: −3, −2, −2, −2, −2, −1





