This is "Unit 8", section 8.1 from the book General Chemistry (v. 1.0).
8.1 Acid–Base Reactions
Learning Objective
- To know the characteristic properties of acids and bases.
In Section 7.2, we learned that practically every chemical reaction can be classified as a precipitation reaction, an acid-base neutralization reaction or a redox reaction. In the last section, section 7.3, we looked at precipitation reactions in depth. In this section we tackle the acid-base neutralization reactions which are essential in both biochemistry and industrial chemistry. Moreover, many of the substances we encounter in our homes, the supermarket, and the pharmacy are acids or bases. For example, aspirin is an acid (acetylsalicylic acid), and antacids are bases. In fact, every amateur chef who has prepared mayonnaise or squeezed a wedge of lemon to marinate a piece of fish has carried out an acid–base reaction. Before we discuss the characteristics of such reactions, let’s first describe some of the properties of acids and bases.
Definitions of Acids and Bases
So far in the story, we defined acids as substances that dissolve in water to produce H+ ions, whereas bases were defined as substances that dissolve in water to produce OH− ions. In fact, this is only one possible set of definitions. Although the general properties of acids and bases have been known for more than a thousand years, the definitions of acid and base have changed dramatically as scientists have learned more about them. In ancient times, an acid was any substance that had a sour taste (e.g., vinegar or lemon juice), caused consistent color changes in dyes derived from plants (e.g., turning blue litmus paper red), reacted with certain metals to produce hydrogen gas and a solution of a salt containing a metal cation, and dissolved carbonate salts such as limestone (CaCO3) with the evolution of carbon dioxide. In contrast, a base was any substance that had a bitter taste, felt slippery to the touch, and caused color changes in plant dyes that differed diametrically from the changes caused by acids (e.g., turning red litmus paper blue). Although these definitions were useful, they were entirely descriptive.

Image Credit: Lemon-By Bordercolliez (Own work) [CC0], via Wikimedia Commons. Soap-By Maksim, https://creativecommons.org/licenses/by-sa/3.0/ via Wikimedia Commons
The Arrhenius Definition of Acids and Bases
The first person to define acids and bases in detail was the Swedish chemist Svante Arrhenius (1859–1927; Nobel Prize in Chemistry, 1903). According to the Arrhenius definition, an acid is a substance like hydrochloric acid that dissolves in water to produce H+ ions (protons; Equation 8.1(eq1)), and a base is a substance like sodium hydroxide that dissolves in water to produce hydroxide (OH−) ions (Equation 8.1(eq2)):
Equation 8.1(eq1)
Equation 8.1(eq2)
According to Arrhenius, the characteristic properties of acids and bases are due exclusively to the presence of H+ and OH− ions, respectively, in solution.
Although Arrhenius’s ideas were widely accepted, his definition of acids and bases had two major limitations. First, because acids and bases were defined in terms of ions obtained from water, the Arrhenius concept applied only to substances in aqueous solution. Second, and more important, the Arrhenius definition predicted that only substances that dissolve in water to produce H+ and OH− ions should exhibit the properties of acids and bases, respectively. For example, according to the Arrhenius definition, the reaction of ammonia (a base) with gaseous HCl (an acid) to give ammonium chloride (Equation 8.1(eq3)) is not an acid–base reaction because it does not involve H+ and OH−:
Equation 8.1(eq3)
NH3(g) + HCl(g) → NH4Cl(s)The Brönsted-Lowry Definition of Acids and Bases
Because of the limitations of the Arrhenius definition, a more general definition of acids and bases was needed. One was proposed independently in 1923 by the Danish chemist J. N. Brönsted (1879–1947) and the British chemist T. M. Lowry (1874–1936), who defined acid–base reactions in terms of the transfer of a proton (H+ ion) from one substance to another.
According to Brönsted and Lowry, an acidA substance with at least one hydrogen atom that can dissociate to form an anion and an ion (a proton) in aqueous solution, thereby foming an acidic solution. is any substance that can donate a proton, and a baseA substance that produces one or more hydroxide ions and a cation when dissolved in aqueous solution, thereby forming a basic solution. is any substance that can accept a proton. The Brönsted-Lowry definition of an acid is essentially the same as the Arrhenius definition, except that it is not restricted to aqueous solutions. The Brönsted–Lowry definition of a base, however, is far more general because the hydroxide ion is just one of many substances that can accept a proton. Ammonia, for example, reacts with a proton to form NH4+, so in Equation 8.1(eq3), NH3 is a Brönsted-Lowry base and HCl is a Brönsted-Lowry acid. Because of its more general nature, the Brönsted-Lowry definition is used throughout this text unless otherwise specified. As one progresses through a sequence of chemistry courses, one sees the definitions of acid and base expand further still.
Polyprotic Acids
Acids differ in the number of protons they can donate. For example, monoprotic acidsA compound that is capable of donating one proton per molecule. are compounds that are capable of donating a single proton per molecule. Monoprotic acids include HF, HCl, HBr, HI, HNO3, and HNO2. All carboxylic acids that contain a single −CO2H group, such as acetic acid (CH3CO2H), are monoprotic acids, dissociating to form RCO2− and H+. Polyprotic acidsA compound that can donate more than one proton per molecule. can donate more than one proton per molecule. For example, H2SO4 can donate two H+ ions in separate steps, so it is a diprotic acidA compound that can donate two protons per molecule in separate steps., and H3PO4, which is capable of donating three protons in successive steps, is a triprotic acidA compound that can donate three protons per molecule in separate steps. (Equation 8.1(eq4), Equation 8.1(eq5), and Equation 8.1(eq6)):
Equation 8.1(eq4)
Equation 8.1(eq5)
Equation 8.1(eq6)
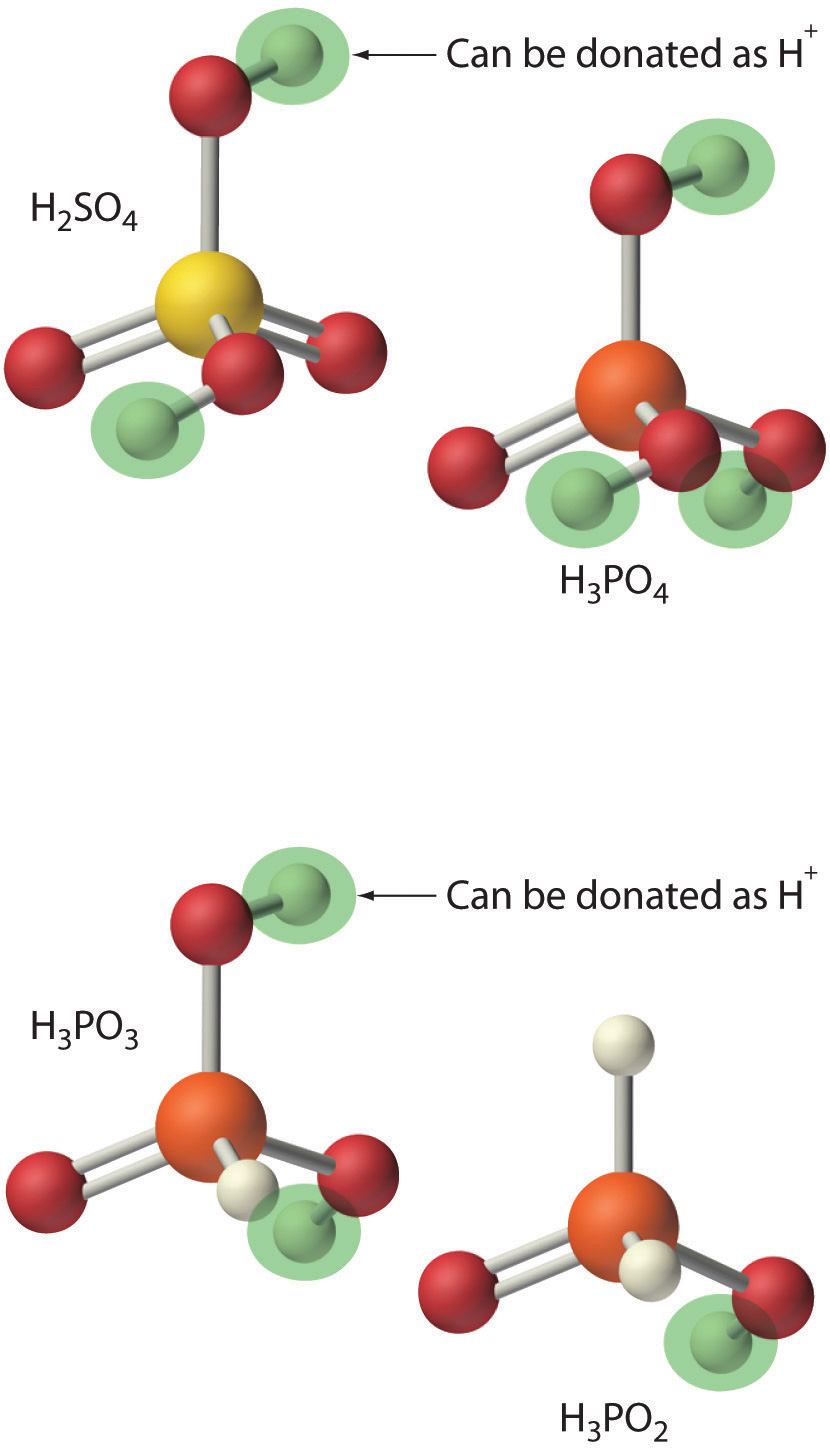
In chemical equations such as these, a double arrow is used to indicate that both the forward and reverse reactions occur simultaneously, so the forward reaction does not go to completion. Instead, the solution contains significant amounts of both reactants and products. Over time, the reaction reaches a state in which the concentration of each species in solution remains constant. The reaction is then said to be in equilibriumThe point at which the rates of the forward and reverse reactions become the same, so that the net composition of the system no longer changes with time. .
Strengths of Acids and Bases
We will not discuss the strengths of acids and bases quantitatively until we explore equilibrium in more depth. Qualitatively, however, we can state that strong acidsAn acid that reacts essentially completely with water to give and the corresponding anion. react essentially completely with water to give H+ and the corresponding anion. Similarly, strong basesA base that dissociates essentially completely in water to give and the corresponding cation. dissociate essentially completely in water to give OH− and the corresponding cation. Strong acids and strong bases are both strong electrolytes. In contrast, only a fraction of the molecules of weak acidsAn acid in which only a fraction of the molecules react with water to produce and the corresponding anion. and weak basesA base in which only a fraction of the molecules react with water to produce and the corresponding cation. react with water to produce ions, so weak acids and weak bases are also weak electrolytes. Typically less than 5% of a weak electrolyte dissociates into ions in solution, whereas more than 95% is present in undissociated form.
In practice, only a few strong acids are commonly encountered: HCl, HBr, HI, HNO3, HClO4, and H2SO4 (H3PO4 is only moderately strong). The most common strong bases are ionic compounds that contain the hydroxide ion as the anion; three examples are NaOH, KOH, and Ca(OH)2. Common weak acids include HCN, H2S, HF, oxoacids such as HNO2 and HClO, and carboxylic acids such as acetic acid. The ionization reaction of acetic acid is as follows:
Equation 8.1(eq7)
Although acetic acid is very soluble in water, almost all of the acetic acid in solution exists in the form of neutral molecules (less than 1% dissociates). Sulfuric acid is unusual in that it is a strong acid when it donates its first proton (Equation 8.1(eq8)) but a weak acid when it donates its second proton (Equation 8.1(eq9)) as indicated by the single and double arrows, respectively:
Equation 8.1(eq8)
Equation 8.1(eq9)
Consequently, an aqueous solution of sulfuric acid contains H+(aq) ions and a mixture of HSO4−(aq) and SO42−(aq) ions but no H2SO4 molecules.
The most common weak base is ammonia, which reacts with water to form small amounts of hydroxide ion:
Equation 8.1(eq10)
Most of the ammonia (>99%) is present in the form of NH3(g). Amines, which are organic analogues of ammonia, are also weak bases, as are ionic compounds that contain anions derived from weak acids (such as S2−).
Table 8.1(1) lists some common strong acids and bases. Acids other than the six common strong acids are almost invariably weak acids. The only common strong bases are the hydroxides of the alkali metals and the heavier alkaline earths (Ca, Sr, and Ba); any other bases you encounter are most likely weak. Remember that there is no correlation between solubility and whether a substance is a strong or a weak electrolyte! Many weak acids and bases are extremely soluble in water.
Note the Pattern
There is no correlation between the solubility of a substance and whether it is a strong electrolyte, a weak electrolyte, or a nonelectrolyte.
Table 8.1(1) Common Strong Acids and Bases
| Strong Acids | Strong Bases | ||
|---|---|---|---|
| Hydrogen Halides | Oxoacids | Group 1 Hydroxides | Hydroxides of the Heavier Group 2 Elements |
| HCl | HNO3 | LiOH | Ca(OH)2 |
| HBr | H2SO4 | NaOH | Sr(OH)2 |
| HI | HClO4 | KOH | Ba(OH)2 |
| RbOH | |||
| CsOH | |||
Example 8.1-1
Classify each compound as a strong acid, a weak acid, a strong base, a weak base, or none of these.
- CH3CH2CO2H
- CH3OH
- Sr(OH)2
- CH3CH2NH2
- HBrO4
Given: compound
Asked for: acid or base strength
Strategy:
A Determine whether the compound is organic or inorganic.
B If inorganic, determine whether the compound is acidic or basic by the presence of dissociable H+ or OH− ions, respectively. If organic, identify the compound as a weak base or a weak acid by the presence of an amine or a carboxylic acid group, respectively. Recall that all polyprotic acids except H2SO4 are weak acids.
Solution:
- A This compound is propionic acid, which is organic. B It contains a carboxylic acid group analogous to that in acetic acid, so it must be a weak acid.
- A CH3OH is methanol, an organic compound that contains the −OH group. B As a covalent compound, it does not dissociate to form the OH− ion. Because it does not contain a carboxylic acid (−CO2H) group, methanol also cannot dissociate to form H+(aq) ions. Thus we predict that in aqueous solution methanol is neither an acid nor a base.
- A Sr(OH)2 is an inorganic compound that contains one Sr2+ and two OH− ions per formula unit. B We therefore expect it to be a strong base, similar to Ca(OH)2.
- A CH3CH2NH2 is an amine (ethylamine), an organic compound in which one hydrogen of ammonia has been replaced by an R group. B Consequently, we expect it to behave similarly to ammonia (Equation 8.1(eq10)), reacting with water to produce small amounts of the OH− ion. Ethylamine is therefore a weak base.
- A HBrO4 is perbromic acid, an inorganic compound. B It is not listed in Table 8.1(1) as one of the common strong acids, but that does not necessarily mean that it is a weak acid. If you examine the periodic table, you can see that Br lies directly below Cl in group 17. We might therefore expect that HBrO4 is chemically similar to HClO4, a strong acid—and, in fact, it is.
Exercise
Classify each compound as a strong acid, a weak acid, a strong base, a weak base, or none of these.
- Ba(OH)2
- HIO4
- CH3CH2CH2CO2H
- (CH3)2NH
- CH2O
Answer:
- strong base
- strong acid
- weak acid
- weak base
- none of these; formaldehyde is a neutral molecule
The Hydronium Ion
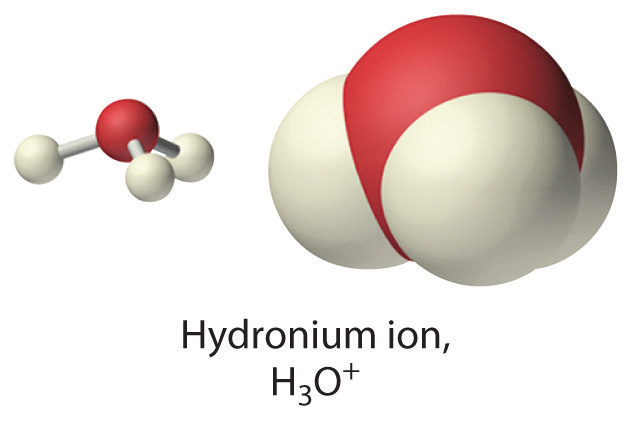
Because isolated protons are very unstable and hence very reactive, an acid never simply “loses” an H+ ion. Instead, the proton is always transferred to another substance, which acts as a base in the Brönsted-Lowry definition. Thus in every acid–base reaction, one species acts as an acid and one species acts as a base. Occasionally, the same substance performs both roles, as you will see later. When a strong acid dissolves in water, the proton that is released is transferred to a water molecule that acts as a proton acceptor or base, as shown for the dissociation of sulfuric acid:
Equation 8.1(eq11)
Technically, therefore, it is imprecise to describe the dissociation of a strong acid as producing H+(aq) ions, as we have been doing. The resulting H3O+ ion, called the hydronium ionThe ion, represented as , is a more accurate representation of H+(aq). For the sake of brevity, however, in discussing acid dissociation reactions, we will often show the product as H+(aq) (as in Equation 8.1(eq7)) with the understanding that the product is actually the H3O+(aq) ion.
Conversely, bases that do not contain the hydroxide ion accept a proton from water, so small amounts of OH− are produced, as in the following:
Equation 8.1(eq12)
Again, the double arrow indicates that the reaction does not go to completion but rather reaches a state of equilibrium. In this reaction, water acts as an acid by donating a proton to ammonia, and ammonia acts as a base by accepting a proton from water. Thus water can act as either an acid or a base by donating a proton to a base or by accepting a proton from an acid. Substances that can behave as both an acid and a base are said to be amphotericWhen substances can behave as both an acid and a base..
The products of an acid–base reaction are also an acid and a base. In Equation 8.1(eq11), for example, the products of the reaction are the hydronium ion, here an acid, and the hydrogen sulfate ion, here a weak base. In Equation 8.1(eq12), the products are NH4+, an acid, and OH−, a base. The product NH4+ is called the conjugate acidThe substance formed when a Brönsted-Lowry base accepts a proton. of the base NH3, and the product OH− is called the conjugate baseThe substance formed when a Brönsted-Lowry acid donates a proton. of the acid H2O. Thus all acid–base reactions actually involve two conjugate acid–base pairsAn acid and a base that differ by only one hydrogen ion. All acid–base reactions involve two conjugate acid–base pairs, the Brönsted-Lowry acid and the base it forms after donating its proton, and the Brönsted-Lowry base and the acid it forms after accepting a proton.; in Equation 8.1(eq12), they are NH4+/NH3 and H2O/OH−.
Neutralization Reactions
A neutralization reactionA chemical reaction in which an acid and a base react in stoichiometric amounts to produce water and a salt. is one in which an acid and a base react in stoichiometric amounts to produce water and a saltThe general term for any ionic substance that does not have as the anion or as the cation., the general term for any ionic substance that does not have OH− as the anion or H+ as the cation. If the base is a metal hydroxide, then the general formula for the reaction of an acid with a base is described as follows: Acid plus base yields water plus salt. For example, the reaction of equimolar amounts of HBr and NaOH to give water and a salt (NaBr) is a neutralization reaction:
Equation 8.1(eq13)
Note the Pattern
Acid plus base yields water plus salt.
The strengths of the acid and the base generally determine whether the reaction goes to completion. The reaction of any strong acid with any strong base goes essentially to completion, as does the reaction of a strong acid with a weak base, and a weak acid with a strong base. Examples of the last two are as follows:
Equation 8.1(eq14)
Equation 8.1(eq15)
Sodium acetate is written with the organic component first followed by the cation, as is usual for organic salts. Most reactions of a weak acid with a weak base also go essentially to completion. One example is the reaction of acetic acid with ammonia:
Equation 8.1(eq16)
An example of an acid–base reaction that does not go to completion is the reaction of a weak acid or a weak base with water, which is both an extremely weak acid and an extremely weak base.
Note the Pattern
Except for the reaction of a weak acid or a weak base with water, acid–base reactions essentially go to completion.
In some cases, the reaction of an acid with an anion derived from a weak acid (such as HS−) produces a gas (in this case, H2S). Because the gaseous product escapes from solution in the form of bubbles, the reverse reaction cannot occur. Therefore, these reactions tend to be forced, or driven, to completion. Examples include reactions in which an acid is added to ionic compounds that contain the HCO3−, CN−, or S2− anions, all of which are driven to completion (Figure 8.1(a) ):
Equation 8.1(eq17)
Equation 8.1(eq18)
Equation 8.1(eq19)
Figure 8.1(a) The Reaction of Dilute Aqueous HNO3 with a Solution of Na2CO3

Note the vigorous formation of gaseous CO2. Image Credit: Highland Community College
The reactions in Equation 8.1(eq19) are responsible for the rotten egg smell that is produced when metal sulfides come in contact with acids.
Example 8.1-2
Calcium propionate is used to inhibit the growth of molds in foods, tobacco, and some medicines. Write a balanced chemical equation for the reaction of aqueous propionic acid (CH3CH2CO2H) with aqueous calcium hydroxide [Ca(OH)2] to give calcium propionate. Do you expect this reaction to go to completion, making it a feasible method for the preparation of calcium propionate?
Given: reactants and product
Asked for: balanced chemical equation and whether the reaction will go to completion
Strategy:
Write the balanced chemical equation for the reaction of propionic acid with calcium hydroxide. Based on their acid and base strengths, predict whether the reaction will go to completion.
Solution:
Propionic acid is an organic compound that is a weak acid, and calcium hydroxide is an inorganic compound that is a strong base. The balanced chemical equation is as follows:
2CH3CH2CO2H(aq) + Ca(OH)2(aq) → (CH3CH2CO2)2Ca(aq) + 2H2O(l)The reaction of a weak acid and a strong base will go to completion, so it is reasonable to prepare calcium propionate by mixing solutions of propionic acid and calcium hydroxide in a 2:1 mole ratio.
Exercise
Write a balanced chemical equation for the reaction of solid sodium acetate with dilute sulfuric acid to give sodium sulfate.
Answer: 2CH3CO2Na(s) + H2SO4(aq) → Na2SO4(aq) + 2CH3CO2H(aq)

Antacid in Space. Expedition 43 Commander and NASA astronaut Terry Virts creates a sphere of bubbles in the station's microgravity environment using drinking water and an antacid tablet. Image catalogued by Johnson Space Center of the United States National Aeronautics and Space Administration (NASA) under Photo ID: ISS043-E-276537. Public Domain, Courtesy NASA/JPL-Caltech. .
One of the most familiar and most heavily advertised applications of acid–base chemistry is antacids, which are bases that neutralize stomach acid. The human stomach contains an approximately 0.1 M solution of hydrochloric acid that helps digest foods. If the protective lining of the stomach breaks down, this acid can attack the stomach tissue, resulting in the formation of an ulcer. Because one factor that is believed to contribute to the formation of stomach ulcers is the production of excess acid in the stomach, many individuals routinely consume large quantities of antacids. The active ingredients in antacids include sodium bicarbonate and potassium bicarbonate (NaHCO3 and KHCO3; Alka-Seltzer); a mixture of magnesium hydroxide and aluminum hydroxide [Mg(OH)2 and Al(OH)3; Maalox, Mylanta]; calcium carbonate (CaCO3; Tums); and a complex salt, dihydroxyaluminum sodium carbonate [NaAl(OH)2CO3; original Rolaids]. Each has certain advantages and disadvantages. For example, Mg(OH)2 is a powerful laxative (it is the active ingredient in milk of magnesia), whereas Al(OH)3 causes constipation. When mixed, each tends to counteract the unwanted effects of the other. Although all antacids contain both an anionic base (OH−, CO32−, or HCO3−) and an appropriate cation, they differ substantially in the amount of active ingredient in a given mass of product.
Summary
Acid–base reactions require both an acid and a base. In Brönsted-Lowry terms, an acid is a substance that can donate a proton (H+), and a base is a substance that can accept a proton. All acid–base reactions contain two acid–base pairs: the reactants and the products. Acids can donate one proton (monoprotic acids), two protons (diprotic acids), or three protons (triprotic acids). Compounds that are capable of donating more than one proton are generally called polyprotic acids. Acids also differ in their tendency to donate a proton, a measure of their acid strength. Strong acids react completely with water to produce H3O+(aq) (the hydronium ion), whereas weak acids dissociate only partially in water. Conversely, strong bases react completely with water to produce the hydroxide ion, whereas weak bases react only partially with water to form hydroxide ions. The reaction of a strong acid with a strong base is a neutralization reaction, which produces water plus a salt.
Key Takeaway
- An acidic solution and a basic solution react together in a neutralization reaction that also forms a salt.
Conceptual Problems
-
Why was it necessary to expand on the Arrhenius definition of an acid and a base? What specific point does the Brönsted-Lowry definition address?
-
State whether each compound is an acid, a base, or a salt.
- CaCO3
- NaHCO3
- H2SO4
- CaCl2
- Ba(OH)2
-
State whether each compound is an acid, a base, or a salt.
- NH3
- NH4Cl
- H2CO3
- CH3COOH
- NaOH
-
Classify each compound as a strong acid, a weak acid, a strong base, or a weak base in aqueous solution.
- sodium hydroxide
- acetic acid
- magnesium hydroxide
- tartaric acid
- sulfuric acid
- ammonia
- hydroxylamine (NH2OH)
- hydrocyanic acid
-
Decide whether each compound forms an aqueous solution that is strongly acidic, weakly acidic, strongly basic, or weakly basic.
- propanoic acid
- hydrobromic acid
- methylamine
- lithium hydroxide
- citric acid
- sodium acetate
- ammonium chloride
- barium hydroxide
-
What is the relationship between the strength of an acid and the strength of the conjugate base derived from that acid? Would you expect the CH3CO2− ion to be a strong base or a weak base? Why? Is the hydronium ion a strong acid or a weak acid? Explain your answer.
-
What are the products of an acid–base reaction? Under what circumstances is one of the products a gas?
-
Explain how an aqueous solution that is strongly basic can have a pH, which is a measure of the acidity of a solution.
Answer
-
The Arrhenius definition requires the reaction involve H+(aq) and OH-(aq). The Brönsted-Lowry definition only requires H+ donors and acceptors. So the Brönsted-Lowry theory extends the Arrhenius definition to include the reaction of ammonia gas with hydrogen chloride gas to form ammonium chloride salt. No water molecules are involved in this reaction.
-
-
- base
- salt (specifically, an acidic salt)
- acid
- acid
- base
-
-
- weakly acidic
- strongly acidic
- weakly basic
- strongly basic
- weakly acidic
- weakly basic
- weakly acidic
- strongly basic
-
-
Always a weaker acid and a weaker base. This pair is often water and a salt.
-
Numerical Problems
-
Given the following salts, identify the acid and the base in the neutralization reactions and then write the complete ionic equation:
- barium sulfate
- lithium nitrate
- sodium bromide
- calcium perchlorate
-
Write the balanced chemical equation for each reaction.
- perchloric acid with potassium hydroxide
- nitric acid with calcium hydroxide
-
Write the balanced chemical equation for each reaction.
- solid strontium hydroxide with hydrobromic acid
- aqueous sulfuric acid with solid sodium hydroxide
-
A neutralization reaction gives calcium nitrate as one of the two products. Identify the acid and the base in this reaction. What is the second product? If the product had been cesium iodide, what would have been the acid and the base?
Answers
-
- H2SO4 and Ba(OH)2; 2H+ + SO42− + Ba2+ + 2OH− → 2H2O + Ba2+ + SO42−
- HNO3 and LiOH; H+ + NO3− + Li+ + OH− → H2O + Li+ + NO3−
- HBr and NaOH; H+ + Br− + Na+ + OH− → H2O + Na+ + Br−
- HClO4 and Ca(OH)2; 2H+ + 2ClO4− + Ca2+ + 2OH− → 2H2O + Ca2+ + 2ClO4−
-
-
- Sr(OH)2(s) + 2HBr(aq) → SrBr2(aq) + 2HOH(l)
- H2SO4(aq) + 2NaOH(s) → Na2SO4(aq) + 2HOH(l)
-
The acid is nitric acid, and the base is calcium hydroxide. The other product is water.
2HNO3 + Ca(OH)2 → Ca(NO3)2 + 2H2OThe acid is hydroiodic acid, and the base is cesium hydroxide. The other product is water.
HI + CsOH → CsI + H2O





