This is “The Alkaline Earth Metals (Group 2)”, section 21.4 from the book Principles of General Chemistry (v. 1.0). For details on it (including licensing), click here.
For more information on the source of this book, or why it is available for free, please see the project's home page. You can browse or download additional books there.
21.4 The Alkaline Earth Metals (Group 2)
Learning Objectives
- To describe how to isolate the alkaline earth metals.
- To be familiar with the reactions, compounds, and complexes of the alkaline earth metals.
Like the alkali metals, the alkaline earth metals are so reactive that they are never found in elemental form in nature. Because they form +2 ions that have very negative reduction potentials, large amounts of energy are needed to isolate them from their ores. Four of the six group 2 elements—magnesium (Mg), calcium (Ca), strontium (Sr), and barium (Ba)—were first isolated in the early 19th century by Sir Humphry Davy, using a technique similar to the one he used to obtain the first alkali metals. In contrast to the alkali metals, however, compounds of the alkaline earth metals had been recognized as unique for many centuries. In fact, the name alkali comes from the Arabic al-qili, meaning “ashes,” which were known to neutralize acids. Medieval alchemists found that a portion of the ashes would melt on heating, and these substances were later identified as the carbonates of sodium and potassium (M2CO3). The ashes that did not melt (but did dissolve in acid), originally called alkaline earths, were subsequently identified as the alkaline earth oxides (MO). In 1808, Davy was able to obtain pure samples of Mg, Ca, Sr, and Ba by electrolysis of their chlorides or oxides.
Beryllium (Be), the lightest alkaline earth metal, was first obtained in 1828 by Friedrich Wöhler in Germany and simultaneously by Antoine Bussy in France. The method used by both men was reduction of the chloride by the potent “new” reductant, potassium:
Equation 21.21
Radium was discovered in 1898 by Pierre and Marie Curie, who processed tons of residue from uranium mines to obtain about 120 mg of almost pure RaCl2. Marie Curie was awarded the Nobel Prize in Chemistry in 1911 for its discovery. Because of its low abundance and high radioactivity however, radium has few uses and will not be discussed further.
Preparation of the Alkaline Earth Metals
The alkaline earth metals are produced for industrial use by electrolytic reduction of their molten chlorides, as indicated in this equation for calcium:
Equation 21.22
CaCl2(l) → Ca(l) + Cl2(g)The group 2 metal chlorides are obtained from a variety of sources. For example, BeCl2 is produced by reacting HCl with beryllia (BeO), which is obtained from the semiprecious stone beryl [Be3Al2(SiO3)6].

A crystal of beryl. Beryl is a gemstone and an important source of beryllium. Locality: Harris Claim, Wah Wah Mountans, Utah Size: small cabinet, 6 x 2.7 x 2.6 cm Red Beryl This is a superb red beryl specimen. It is considered by many to be one of the finest and most unique American mineral specimens in existence. It was mined and sold directly to prominent collector F. John Barlow in the early 1990s (and is listed in his book, page 357, as the world's foremost example of the species). He had a core suite of 14 remarkable specimens of which this was the most important, and spent a fortune keeping on top of the finds here to have the best assemblage possible from this unique site. The locality is currently defunct but until recently was attracting the attention of gemstone giants like Tiffany's for its novel mineral. This particular piece is featured prominently in many media, including the F. John Barlow Collection Book, Lapis special issues on beryls, and probably any other work that references red beryl. Although it "disappeared" briefly and could not make the American Mineral Treasures exhibition in Tucson in 2008, it well should have been in that compendium case. However, the photo was still chosen as the lead specimen for the Red Beryl chapter of the companion book to that monumental exhibition, and is shown full-page on page 217 of American Mineral Treasures. The crystal is 2 inches.Image and caption credit: Rob Lavinsky, iRocks.com – CC-BY-SA-3.0 [CC BY-SA 3.0 (http://creativecommons.org/licenses/by-sa/3.0)], via Wikimedia Commons
Chemical reductants can also be used to obtain the group 2 elements. For example, magnesium is produced on a large scale by heating a form of limestone called dolomite (CaCO3·MgCO3) with an inexpensive iron/silicon alloy at 1150°C. Initially CO2 is released, leaving behind a mixture of CaO and MgO; Mg2+ is then reduced:
Equation 21.23
2CaO·MgO(s) + Fe/Si(s) → 2Mg(l) + Ca2SiO4(s) + Fe(s)An early source of magnesium was an ore called magnesite (MgCO3) from the district of northern Greece called Magnesia. Strontium was obtained from strontianite (SrCO3) found in a lead mine in the town of Strontian in Scotland. The alkaline earth metals are somewhat easier to isolate from their ores, as compared to the alkali metals, because their carbonate and some sulfate and hydroxide salts are insoluble.

A crystal of strontianite. Both strontianite, one of the most important strontium ores, and strontium are named after the town of Strontian, Scotland, the location of one of the first mines for strontium ores. Image Credit: Rob Lavinsky, iRocks.com – CC-BY-SA-3.0 [CC BY-SA 3.0 (http://creativecommons.org/licenses/by-sa/3.0)], via Wikimedia Commons
General Properties of the Alkaline Earth Metals
Several important properties of the alkaline earth metals are summarized in Table 21.4 "Selected Properties of the Group 2 Elements". Although many of these properties are similar to those of the alkali metals (Table 21.3 "Selected Properties of the Group 1 Elements"), certain key differences are attributable to the differences in the valence electron configurations of the two groups (ns2 for the alkaline earth metals versus ns1 for the alkali metals).
Table 21.4 Selected Properties of the Group 2 Elements
| Beryllium | Magnesium | Calcium | Strontium | Barium | Radium | |
|---|---|---|---|---|---|---|
| atomic symbol | Be | Mg | Ca | Sr | Ba | Ra |
| atomic number | 4 | 12 | 20 | 38 | 56 | 88 |
| atomic mass | 9.01 | 24.31 | 40.08 | 87.62 | 137.33 | 226 |
| valence electron configuration | 2s2 | 3s2 | 4s2 | 5s2 | 6s2 | 7s2 |
| melting point/boiling point (°C) | 1287/2471 | 650/1090 | 842/1484 | 777/1382 | 727/1897 | 700/— |
| density (g/cm3) at 25°C | 1.85 | 1.74 | 1.54 | 2.64 | 3.62 | ∼5 |
| atomic radius (pm) | 112 | 145 | 194 | 219 | 253 | — |
| first ionization energy (kJ/mol) | 900 | 738 | 590 | 549 | 503 | — |
| most common oxidation state | +2 | +2 | +2 | +2 | +2 | +2 |
| ionic radius (pm)* | 45 | 72 | 100 | 118 | 135 | — |
| electron affinity (kJ/mol) | ≥ 0 | ≥ 0 | −2 | −5 | −14 | — |
| electronegativity | 1.6 | 1.3 | 1.0 | 1.0 | 0.9 | 0.9 |
| standard electrode potential (E°, V) | −1.85 | −2.37 | −2.87 | −2.90 | −2.91 | −2.8 |
| product of reaction with O2 | BeO | MgO | CaO | SrO | BaO2 | — |
| type of oxide | amphoteric | weakly basic | basic | basic | basic | — |
| product of reaction with N2 | none | Mg3N2 | Ca3N2 | Sr3N2 | Ba3N2 | — |
| product of reaction with X2 | BeX2 | MgX2 | CaX2 | SrX2 | BaX2 | — |
| product of reaction with H2 | none | MgH2 | CaH2 | SrH2 | BaH2 | — |
| *The values cited are for six-coordinate ions except for Be2+, for which the value for the four-coordinate ion is given. | ||||||
As with the alkali metals, the atomic and ionic radii of the alkaline earth metals increase smoothly from Be to Ba, and the ionization energies decrease. As we would expect, the first ionization energy of an alkaline earth metal, with an ns2 valence electron configuration, is always significantly greater than that of the alkali metal immediately preceding it. The group 2 elements do exhibit some anomalies, however. For example, the density of Ca is less than that of Be and Mg, the two lightest members of the group, and Mg has the lowest melting and boiling points. In contrast to the alkali metals, the heaviest alkaline earth metal (Ba) is the strongest reductant, and the lightest (Be) is the weakest. The standard electrode potentials of Ca and Sr are not very different from that of Ba, indicating that the opposing trends in ionization energies and hydration energies are of roughly equal importance.
One major difference between the group 1 and group 2 elements is their electron affinities. With their half-filled ns orbitals, the alkali metals have a significant affinity for an additional electron. In contrast, the alkaline earth metals generally have little or no tendency to accept an additional electron because their ns valence orbitals are already full; an added electron would have to occupy one of the vacant np orbitals, which are much higher in energy.
Reactions and Compounds of the Alkaline Earth Metals
With their low first and second ionization energies, the group 2 elements almost exclusively form ionic compounds that contain M2+ ions. As expected, however, the lightest element (Be), with its higher ionization energy and small size, forms compounds that are largely covalent, as discussed in Section 21.1 "Overview of Periodic Trends". Some compounds of Mg2+ also have significant covalent character. Hence organometallic compounds like those discussed for Li in group 1 are also important for Be and Mg in group 2.
Note the Pattern
The group 2 elements almost exclusively form ionic compounds containing M2+ ions.
Note the Pattern
Because of their higher ionization energy and small size, both Be and Mg form organometallic compounds.
All alkaline earth metals react vigorously with the halogens (group 17) to form the corresponding halides (MX2). Except for the beryllium halides, these compounds are all primarily ionic in nature, containing the M2+ cation and two X− anions. The beryllium halides, with properties more typical of covalent compounds, have a polymeric halide-bridged structure in the solid state, as shown for BeCl2. These compounds are volatile, producing vapors that contain the linear X–Be–X molecules predicted by the valence-shell electron-pair repulsion (VSEPR) model. As expected for compounds with only four valence electrons around the central atom, the beryllium halides are potent Lewis acids. They react readily with Lewis bases, such as ethers, to form tetrahedral adducts in which the central beryllium is surrounded by an octet of electrons:
Equation 21.24
BeCl2(s) + 2(CH3CH2)2O(l) → BeCl2[O(CH2CH3)2]2(soln)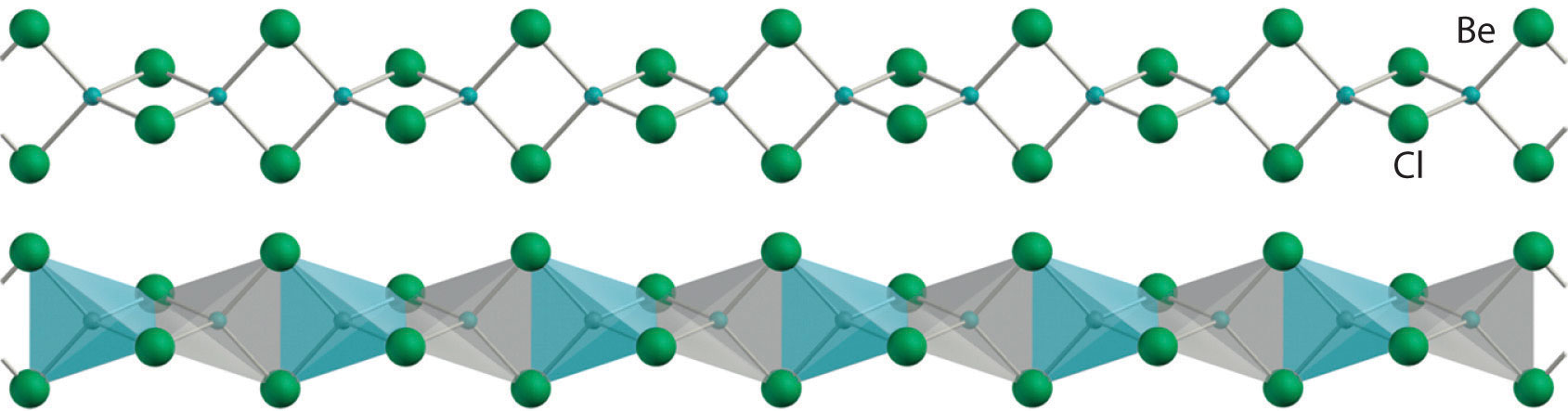
Solid beryllium chloride (BeCl2). The solid has a polymeric, halide-bridged structure.
The reactions of the alkaline earth metals with oxygen are less complex than those of the alkali metals. All group 2 elements except barium react directly with oxygen to form the simple oxide MO. Barium forms barium peroxide (BaO2) because the larger O22− ion is better able to separate the large Ba2+ ions in the crystal lattice. In practice, only BeO is prepared by direct reaction with oxygen, and this reaction requires finely divided Be and high temperatures because Be is relatively inert. The other alkaline earth oxides are usually prepared by the thermal decomposition of carbonate salts:
Equation 21.25
The reactions of the alkaline earth metals with the heavier chalcogens (Y) are similar to those of the alkali metals. When the reactants are present in a 1:1 ratio, the binary chalcogenides (MY) are formed; at lower M:Y ratios, salts containing polychalcogenide ions (Yn2−) are formed.
In the reverse of Equation 21.25, the oxides of Ca, Sr, and Ba react with CO2 to regenerate the carbonate. Except for BeO, which has significant covalent character and is therefore amphoteric, all the alkaline earth oxides are basic. Thus they react with water to form the hydroxides—M(OH)2:
Equation 21.26
MO(s) + H2O(l) → M2+(aq) + 2OH−(aq)and they dissolve in aqueous acid. Hydroxides of the lighter alkaline earth metals are insoluble in water, but their solubility increases as the atomic number of the metal increases. Because BeO and MgO are much more inert than the other group 2 oxides, they are used as refractory materials in applications involving high temperatures and mechanical stress. For example, MgO (melting point = 2825°C) is used to coat the heating elements in electric ranges.
The carbonates of the alkaline earth metals also react with aqueous acid to give CO2 and H2O:
Equation 21.27
MCO3(s) + 2H+(aq) → M2+(aq) + CO2(g) + H2O(l)The reaction in Equation 21.27 is the basis of antacids that contain MCO3, which is used to neutralize excess stomach acid.
The trend in the reactivities of the alkaline earth metals with nitrogen is the opposite of that observed for the alkali metals. Only the lightest element (Be) does not react readily with N2 to form the nitride (M3N2), although finely divided Be will react at high temperatures. The higher lattice energy due to the highly charged M2+ and N3− ions is apparently sufficient to overcome the chemical inertness of the N2 molecule, with its N≡N bond. Similarly, all the alkaline earth metals react with the heavier group 15 elements to form binary compounds such as phosphides and arsenides with the general formula M3Z2.
Note the Pattern
Higher lattice energies cause the alkaline earth metals to be more reactive than the alkali metals toward group 15 elements.
When heated, all alkaline earth metals, except for beryllium, react directly with carbon to form ionic carbides with the general formula MC2. The most important alkaline earth carbide is calcium carbide (CaC2), which reacts readily with water to produce acetylene. For many years, this reaction was the primary source of acetylene for welding and lamps on miners’ helmets. In contrast, beryllium reacts with elemental carbon to form Be2C, which formally contains the C4− ion (although the compound is covalent). Consistent with this formulation, reaction of Be2C with water or aqueous acid produces methane:
Equation 21.28
Be2C(s) + 4H2O(l) → 2Be(OH)2(s) + CH4(g)Beryllium does not react with hydrogen except at high temperatures (1500°C), although BeH2 can be prepared at lower temperatures by an indirect route. All the heavier alkaline earth metals (Mg through Ba) react directly with hydrogen to produce the binary hydrides (MH2). The hydrides of the heavier alkaline earth metals are ionic, but both BeH2 and MgH2 have polymeric structures that reflect significant covalent character. All alkaline earth hydrides are good reducing agents that react rapidly with water or aqueous acid to produce hydrogen gas:
Equation 21.29
CaH2(s) + 2H2O(l) → Ca(OH)2(s) + 2H2(g)Like the alkali metals, the heavier alkaline earth metals are sufficiently electropositive to dissolve in liquid ammonia. In this case, however, two solvated electrons are formed per metal atom, and no equilibriums involving metal dimers or metal anions are known. Also, like the alkali metals, the alkaline earth metals form a wide variety of simple ionic salts with oxoanions, such as carbonate, sulfate, and nitrate. The nitrate salts tend to be soluble, but the carbonates and sulfates of the heavier alkaline earth metals are quite insoluble because of the higher lattice energy due to the doubly charged cation and anion. The solubility of the carbonates and the sulfates decreases rapidly down the group because hydration energies decrease with increasing cation size.
Note the Pattern
The solubility of alkaline earth carbonate and sulfates decrease down the group because the hydration energies decrease.
Complexes of the Alkaline Earth Metals
Because of their higher positive charge (+2) and smaller ionic radii, the alkaline earth metals have a much greater tendency to form complexes with Lewis bases than do the alkali metals. This tendency is most important for the lightest cation (Be2+) and decreases rapidly with the increasing radius of the metal ion.
Note the Pattern
The alkaline earth metals have a substantially greater tendency to form complexes with Lewis bases than do the alkali metals.
The chemistry of Be2+ is dominated by its behavior as a Lewis acid, forming complexes with Lewis bases that produce an octet of electrons around beryllium. For example, Be2+ salts dissolve in water to form acidic solutions that contain the tetrahedral [Be(H2O)4]2+ ion. Because of its high charge-to-radius ratio, the Be2+ ion polarizes coordinated water molecules, thereby increasing their acidity:
Equation 21.30
[Be(H2O)4]2+(aq) → [Be(H2O)3(OH)]+(aq) + H+(aq)Similarly, in the presence of a strong base, beryllium and its salts form the tetrahedral hydroxo complex: [Be(OH)4]2−. Hence beryllium oxide is amphoteric. Beryllium also forms a very stable tetrahedral fluoride complex: [BeF4]2−. Recall that beryllium halides behave like Lewis acids by forming adducts with Lewis bases (Equation 21.24).
The heavier alkaline earth metals also form complexes, but usually with a coordination number of 6 or higher. Complex formation is most important for the smaller cations (Mg2+ and Ca2+). Thus aqueous solutions of Mg2+ contain the octahedral [Mg(H2O)6]2+ ion. Like the alkali metals, the alkaline earth metals form complexes with neutral cyclic ligands like the crown ethers and cryptands discussed in the previous section.
Organometallic Compounds Containing Group 2 Elements
Like the alkali metals, the lightest alkaline earth metals (Be and Mg) form the most covalent-like bonds with carbon, and they form the most stable organometallic compounds. Organometallic compounds of magnesium with the formula RMgX, where R is an alkyl or aryl group and X is a halogen, are universally called Grignard reagents, after Victor Grignard (1871–1935), the French chemist who discovered them. (For more information on the Grignard reagents, see Section 24.5 "Common Classes of Organic Compounds".) Grignard reagents can be used to synthesize various organic compounds, such as alcohols, aldehydes, ketones, carboxylic acids, esters, thiols, and amines.
Uses of the Alkaline Earth Metals
Elemental magnesium is the only alkaline earth metal that is produced on a large scale (about 5 × 105 tn per year). Its low density (1.74 g/cm3 compared with 7.87 g/cm3 for iron and 2.70 g/cm3 for aluminum) makes it an important component of the lightweight metal alloys used in aircraft frames and aircraft and automobile engine parts (Figure 21.13 "Magnesium Alloys Are Lightweight and Corrosion Resistant"). Most commercial aluminum actually contains about 5% magnesium to improve its corrosion resistance and mechanical properties. Elemental magnesium also serves as an inexpensive and powerful reductant for the production of a number of metals, including titanium, zirconium, uranium, and even beryllium, as shown in the following equation:
Equation 21.31
TiCl4(l) + 2Mg(s) → Ti(s) + 2MgCl2(s)Figure 21.13 Magnesium Alloys Are Lightweight and Corrosion Resistant

Because magnesium is about five times lighter than steel and 50% lighter than aluminum, it was long considered the “material of the future.” Motorcycle engine blocks cast from magnesium alloys. Image Credit: CSIRO [CC BY 3.0 (http://creativecommons.org/licenses/by/3.0)], via Wikimedia Commons
The only other alkaline earth that is widely used as the metal is beryllium, which is extremely toxic. Ingestion of beryllium or exposure to beryllium-containing dust causes a syndrome called berylliosis, characterized by severe inflammation of the respiratory tract or other tissues. A small percentage of beryllium dramatically increases the strength of copper or nickel alloys, which are used in nonmagnetic, nonsparking tools (such as wrenches and screwdrivers), camera springs, and electrical contacts. The low atomic number of beryllium gives it a very low tendency to absorb x-rays and makes it uniquely suited for applications involving radioactivity. Both elemental Be and BeO, which is a high-temperature ceramic, are used in nuclear reactors, and the windows on all x-ray tubes and sources are made of beryllium foil.
Millions of tons of calcium compounds are used every year. As discussed in earlier chapters, CaCl2 is used as “road salt” to lower the freezing point of water on roads in cold temperatures. In addition, CaCO3 is a major component of cement and an ingredient in many commercial antacids. “Quicklime” (CaO), produced by heating CaCO3 (Equation 21.25), is used in the steel industry to remove oxide impurities, make many kinds of glass, and neutralize acidic soil. Other applications of group 2 compounds described in earlier chapters include the medical use of BaSO4 in “barium milkshakes” for identifying digestive problems by x-rays and the use of various alkaline earth compounds to produce the brilliant colors seen in fireworks.
Example 4
For each application, choose the most appropriate substance based on the properties and reactivities of the alkaline earth metals and their compounds. Explain your choice in each case. Use any tables you need in making your decision, such as Ksp values (Table 17.1 "Solubility Products for Selected Ionic Substances at 25°C"), lattice energies (Table 8.1 "Representative Calculated Lattice Energies"), and band-gap energies (Section 12.6 "Bonding in Metals and Semiconductors").
- To neutralize excess stomach acid that causes indigestion, would you use BeCO3, CaCO3, or BaCO3?
- To remove CO2 from the atmosphere in a space capsule, would you use MgO, CaO, or BaO?
- As a component of the alloy in an automotive spark plug electrode, would you use Be, Ca, or Ba?
Given: application and selected alkaline earth metals
Asked for: most appropriate substance for each application
Strategy:
Based on the discussion in this section and any relevant information elsewhere in this book, determine which substance is most appropriate for the indicated use.
Solution:
- All the alkaline earth carbonates will neutralize an acidic solution by Equation 21.27. Because beryllium and its salts are toxic, however, BeCO3 cannot be used as an antacid. Of the remaining choices, CaCO3 is somewhat more soluble than BaCO3 (according to the Ksp values in Table 17.1 "Solubility Products for Selected Ionic Substances at 25°C"), suggesting that it will act more rapidly. Moreover, the formula mass of CaCO3 is 100.1 amu, whereas that of BaCO3 is almost twice as large. Therefore, neutralizing a given amount of acid would require twice the mass of BaCO3 compared with CaCO3. Furthermore, reaction of BaCO3 with acid produces a solution containing Ba2+ ions, which are toxic. (Ba2+ is a stimulant that can cause ventricular fibrillation of the heart.) Finally, CaCO3 is produced on a vast scale, so CaCO3 is likely to be significantly less expensive than any barium compound. Consequently, CaCO3 is the best choice for an antacid.
- This application involves reacting CO2 with an alkaline earth oxide to form the carbonate, which is the reverse of the thermal decomposition reaction in which MCO3 decomposes to CO2 and the metal oxide MO (Equation 21.25). Owing to their higher lattice energies, the smallest alkaline earth metals should form the most stable oxides. Hence their carbonates should decompose at the lowest temperatures, as is observed (BeCO3 decomposes at 100°C; BaCO3 at 1360°C). If the carbonate with the smallest alkaline earth metal decomposes most readily, we would expect the reverse reaction (formation of a carbonate) to occur most readily with the largest metal cation (Ba2+). Hence BaO is the best choice.
- The alloy in a spark plug electrode must release electrons and promote their flow across the gap between the electrodes at high temperatures. Of the three metals listed, Ba has the lowest ionization energy and thus releases electrons most readily. Heating a barium-containing alloy to high temperatures will cause some ionization to occur, providing the initial step in forming a spark.
Exercise
Which of the indicated alkaline earth metals or their compounds is most appropriate for each application?
- drying agent for removing water from the atmosphere—CaCl2, MgSO4, or BaF2
- removal of scale deposits (largely CaCO3) in water pipes—HCl(aq) or H2SO4(aq)
- removal of traces of N2 from purified argon gas—Be, Ca, or Ba
Answer:
- MgSO4
- HCl
- Ba
Example 5
Predict the products of each reaction and then balance each chemical equation.
- CaO(s) + HCl(g) →
- MgO(s) + excess OH−(aq) →
Given: reactants
Asked for: products and balanced chemical equation
Strategy:
Follow the procedure given in Example 3 to predict the products of each reaction and then balance each chemical equation.
Solution:
-
A Gaseous HCl is an acid, and CaO is a basic oxide that contains the O2− ion. This is therefore an acid–base reaction that produces CaCl2 and H2O.
B The balanced chemical equation is CaO(s) + 2HCl(g) → CaCl2(aq) + H2O(l).
-
A Magnesium oxide is a basic oxide, so it can either react with water to give a basic solution or dissolve in an acidic solution. Hydroxide ion is also a base. Because we have two bases but no acid, an acid–base reaction is impossible. A redox reaction is not likely because MgO is neither a good oxidant nor a good reductant.
B We conclude that no reaction occurs.
-
A Because CaH2 contains the hydride ion (H−), it is a good reductant. It is also a strong base because H− ions can react with H+ ions to form H2. Titanium oxide (TiO2) is a metal oxide that contains the metal in its highest oxidation state (+4 for a group 4 metal); it can act as an oxidant by accepting electrons. We therefore predict that a redox reaction will occur, in which H− is oxidized and Ti4+ is reduced. The most probable reduction product is metallic titanium, but what is the oxidation product? Oxygen must appear in the products, and both CaO and H2O are stable compounds. The +1 oxidation state of hydrogen in H2O is a sign that an oxidation has occurred (2H− → 2H+ + 4e−).
B The balanced chemical equation is We could also write the products as Ti(s) + Ca(OH)2(s).
Exercise
Predict the products of each reaction and then balance each chemical equation.
- BaCl2(aq) + Na2SO4(aq) →
- BeO(s) + OH−(aq) + H2O(l) →
Answer:
- BaCl2(aq) + Na2SO4(aq) → BaSO4(s) + 2NaCl(aq)
- BeO(s) + 2OH−(aq) + H2O(l) → [Be(OH)4]2−(aq)
Summary
Pure samples of most of the alkaline earth metals can be obtained by electrolysis of the chlorides or oxides. Beryllium was first obtained by the reduction of its chloride; radium chloride, which is radioactive, was obtained through a series of reactions and separations. In contrast to the alkali metals, the alkaline earth metals generally have little or no affinity for an added electron. All alkaline earth metals react with the halogens to produce the corresponding halides, with oxygen to form the oxide (except for barium, which forms the peroxide), and with the heavier chalcogens to form chalcogenides or polychalcogenide ions. All oxides except BeO react with CO2 to form carbonates, which in turn react with acid to produce CO2 and H2O. Except for Be, all the alkaline earth metals react with N2 to form nitrides, and all react with carbon and hydrogen to form carbides and hydrides. Alkaline earth metals dissolve in liquid ammonia to give solutions that contain two solvated electrons per metal atom. The alkaline earth metals have a greater tendency than the alkali metals to form complexes with crown ethers, cryptands, and other Lewis bases. The most important alkaline earth organometallic compounds are Grignard reagents (RMgX), which are used to synthesize organic compounds.
Key Takeaway
- Group 2 elements almost exclusively form ionic compounds containing the M2+ ion, they are more reactive toward group 15 elements, and they have a greater tendency to form complexes with Lewis bases than do the alkali metals.
Conceptual Problems
-
The electronegativities of Li and Sr are nearly identical (0.98 versus 0.95, respectively). Given their positions in the periodic table, how do you account for this?
-
Arrange Na, Ba, Cs, and Li in order of increasing Zeff.
-
Do you expect the melting point of NaCl to be greater than, equal to, or less than that of MgCl2? Why?
-
Which of the group 2 elements would you expect to form primarily ionic rather than covalent organometallic compounds? Explain your reasoning.
-
Explain why beryllium forms compounds that are best regarded as covalent in nature, whereas the other elements in group 2 generally form ionic compounds.
-
Why is the trend in the reactions of the alkaline earth metals with nitrogen the reverse of the trend seen for the alkali metals?
-
Is the bonding in the alkaline earth hydrides primarily ionic or covalent in nature? Explain your answer. Given the type of bonding, do you expect the lighter or heavier alkaline earth metals to be better reducing agents?
-
Using arguments based on ionic size, charge, and chemical reactivity, explain why beryllium oxide is amphoteric. What element do you expect to be most similar to beryllium in its reactivity? Why?
-
Explain why the solubility of the carbonates and sulfates of the alkaline earth metals decreases with increasing cation size.
-
Beryllium oxide is amphoteric, magnesium oxide is weakly basic, and calcium oxide is very basic. Explain how this trend is related to the ionic character of the oxides.
-
Do you expect the of CaH2 to be greater than, the same as, or less than that of BaH2? Why or why not?
-
Which of the s-block elements would you select to carry out a chemical reduction on a small scale? Consider cost, reactivity, and stability in making your choice. How would your choice differ if the reduction were carried out on an industrial scale?
Structure and Reactivity
-
Beryllium iodide reacts vigorously with water to produce HI. Write a balanced chemical equation for this reaction and explain why it is violent.
-
Predict the products of each reaction and then balance each chemical equation.
- Mg(OH)2(aq) + (NH4)3PO4(aq) →
- calcium carbonate and sulfuric acid →
- CaCl2(aq) + Na3PO4(aq) →
- the thermal decomposition of SrCO3
-
Predict the products of each reaction and then balance each chemical equation.
- Sr(s) + O2(g) →
- the thermal decomposition of CaCO3(s)
- CaC2(s) + H2O(l) →
- RbHCO3(s) + H2SO4(aq) →
-
Indicate whether each pair of substances will react and, if so, write a balanced chemical equation for the reaction.
- an alkyl chloride and magnesium metal
- strontium metal and nitrogen
- magnesium metal and cold water
- beryllium and nitrogen
-
Using a thermodynamic cycle, calculate the lattice energy of magnesium nitride (Mg3N2). ( for Mg3N2 is −463 kJ/mol, and ΔH° for N(g) + 3e− → N3− is +1736 kJ.) How does the lattice energy of Mg3N2 compare with that of MgCl2 and MgO? (See Chapter 25 "Appendix A: Standard Thermodynamic Quantities for Chemical Substances at 25°C" for the enthalpy of formation values.)
-
The solubility products of the carbonate salts of magnesium, calcium, and strontium are 6.82 × 10−6, 3.36 × 10−9, and 5.60 × 10−10, respectively. How many milligrams of each compound would be present in 200.0 mL of a saturated solution of each? How would the solubility depend on the pH of the solution? Why?
-
The solubility products of BaSO4 and CaSO4 are 1.08 × 10−10 and 4.93 × 10−5, respectively. What accounts for this difference? When 500.0 mL of a solution that contains 1.00 M Ba(NO3)2 and 3.00 M Ca(NO3)2 is mixed with a 2.00 M solution of Na2SO4, a precipitate forms. What is the identity of the precipitate? How much of it will form before the second salt precipitates?
-
Electrolytic reduction is used to produce magnesium metal from MgCl2. The goal is to produce 200.0 kg of Mg by this method.
- How many kilograms of MgCl2 are required?
- How many liters of chlorine gas will be released at standard temperature and pressure?
- How many hours will it take to process the magnesium metal if a total current of 1.00 × 104 A is used?
-
A sample consisting of 20.35 g of finely divided calcium metal is allowed to react completely with nitrogen. What is the mass of the product?
-
What mass of magnesium hydride will react with water to produce 1.51 L of hydrogen gas at standard temperature and pressure?
Answers
-
-
-
- 2Sr(s) + O2(g) → 2SrO(s)
- CaC2(s) + 2H2O(l) → C2H2(g) + Ca(OH)2(aq)
- 2RbHCO3(s) + H2SO4(aq) → Rb2SO4(aq) + 2CO2(g) + 2H2O(l)
-
-
-
-
The Ba2+ ion is larger and has a lower hydration energy than the Ca2+ ion. The precipitate is BaSO4; 117 g of BaSO4.
-
-
25.09 g of Ca3N2
-




