This is “End-of-Chapter Material”, section 15.8 from the book Principles of General Chemistry (v. 1.0). For details on it (including licensing), click here.
For more information on the source of this book, or why it is available for free, please see the project's home page. You can browse or download additional books there.
15.8 End-of-Chapter Material
Application Problems
-
♦ The total concentrations of dissolved Al in a soil sample represent the sum of “free” Al3+ and bound forms of Al that are stable enough to be considered definite chemical species. The distribution of aluminum among its possible chemical forms can be described using equilibrium constants such as the following:
K1 = [AlOH2+]/[Al3+][OH−] = 1.0 × 109 K2 = [AlSO4+]/[Al3+][SO42−] = 1.0 × 103 K3 = [AlF2+]/[Al3+][F−] = 1.0 × 107- Write an equilibrium equation for each expression.
- Which anion has the highest affinity for Al3+: OH−, SO42−, or F−? Explain your reasoning.
- A 1.0 M solution of Al3+ is mixed with a 1.0 M solution of each of the anions. Which mixture has the lowest Al3+ concentration?
-
Many hydroxy acids form lactones (cyclic esters) that contain a 5- or 6-membered ring. Common hydroxy acids found in nature are glycolic acid, a constituent of cane sugar juice; lactic acid, which has the characteristic odor and taste of sour milk; and citric acid, found in fruit juices. The general reaction for lactone formation can be written as follows:
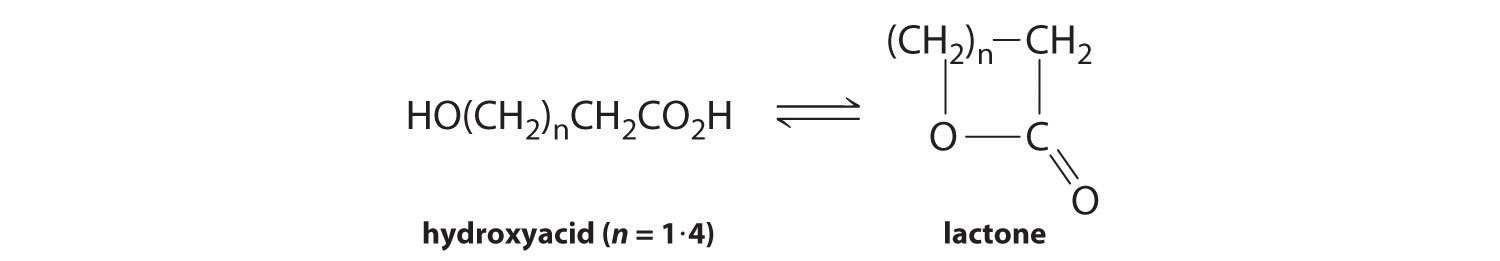
Use the information in the following table to calculate the equilibrium constant for lactone formation for each hydroxy acid given and determine which ring size is most stable.
At Equilibrium Hydroxy Acid Formula Size of Lactone Ring (atoms) Hydroxy Acid (M) Lactone (M) HOCH2CH2COOH 4 4.99 × 10−3 5.00 × 10−5 HOCH2CH2CH2COOH 5 8.10 × 10−5 2.19 × 10−4 HOCH2CH2CH2CH2COOH 6 5.46 × 10−2 5.40 × 10−9 HOCH2CH2CH2CH2CH2COOH 7 9.90 × 10−3 1.00 × 10−4 -
♦ Phosphorus pentachloride, an important reagent in organic chemistry for converting alcohols to alkyl chlorides (ROH → RCl), is hydrolyzed in water to form phosphoric acid and hydrogen chloride. In the gaseous state, however, PCl5 can decompose at 250°C according to for which K = 0.0420.
- Are products or reactants favored in the decomposition of PCl5(g)?
- If a 2.00 L flask containing 104.1 g PCl5 is heated to 250°C, what is the equilibrium concentration of each species in this reaction?
- What effect would an increase in pressure have on the equilibrium position? Why?
- If a 1.00 × 103 L vessel containing 2.00 × 103 kg of PCl3 with a constant chlorine pressure of 2.00 atm is allowed to reach equilibrium, how many kilograms of PCl5 are produced? What is the percent yield of PCl5?
-
♦ Carbon disulfide (CS2) is used in the manufacture of rayon and in electronic vacuum tubes. However, extreme caution must be used when handling CS2 in its gaseous state because it is extremely toxic and can cause fatal convulsions. Chronic toxicity is marked by psychic disturbances and tremors. CS2 is used to synthesize H2S at elevated T via the following reaction:
- If the equilibrium concentration of methane in this reaction is 2.5 × 10−2 M and the initial concentration of each reactant is 0.1635 M, what is the concentration of H2S at equilibrium?
- Exposure to CS2 concentrations greater than 300 ppm for several hours can start to produce adverse effects. After working for several hours in a laboratory that contains large quantities of CS2, you notice that the fume hoods were off and there was not enough ventilation to remove any CS2 vapor. Given the equilibrium where T = 20°C and Kp = 0.391, determine whether you are in any danger.
-
♦ Chloral hydrate, a sedative commonly referred to as “knockout drops,” is in equilibrium with trichloroacetaldehyde in highly concentrated aqueous solutions:
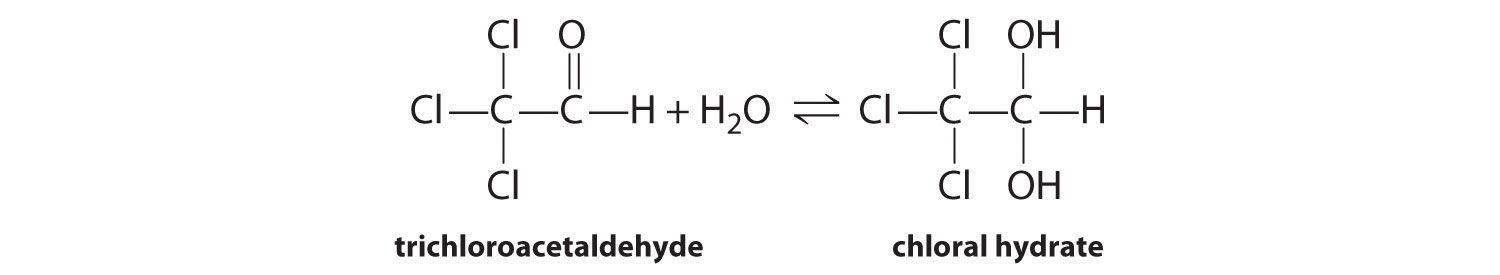
The equilibrium constant for this reaction as written is 3 × 104. Are the products or the reactants favored? Write an equilibrium expression for this reaction. How could you drive this reaction to completion?
-
Hydrogen cyanide is commercially produced in the United States by the following reaction: where HCN is continuously removed from the system. This reaction is carried out at approximately 1100°C in the presence of a catalyst; however, the high temperature causes other reactions to occur. Why is it necessary to run this reaction at such an elevated temperature? Does the presence of the catalyst affect the equilibrium position?
-
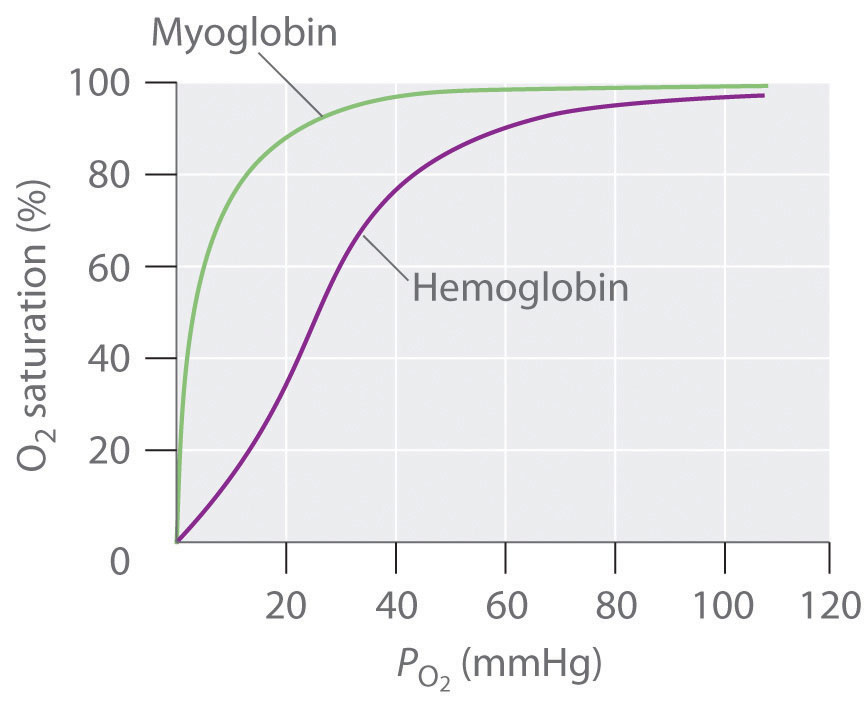
♦ Hemoglobin transports oxygen from the lungs to the capillaries and consists of four subunits, each capable of binding a single molecule of O2. In the lungs, is relatively high (100 mmHg), so hemoglobin becomes nearly saturated with O2. In the tissues, however, is relatively low (40 mmHg), so hemoglobin releases about half of its bound oxygen. Myoglobin, a protein in muscle, resembles a single subunit of hemoglobin. The plots show the percent O2 saturation versus for hemoglobin and myoglobin. Based on these plots, which molecule has the higher affinity for oxygen? What advantage does hemoglobin have over myoglobin as the oxygen transporter? Why is it advantageous to have myoglobin in muscle tissue? Use equilibrium to explain why it is more difficult to exercise at high altitudes where the partial pressure of oxygen is lower.
-
♦ Sodium sulfate is widely used in the recycling industry as well as in the detergent and glass industries. This compound combines with H2SO4 via Sodium hydrogen sulfate is used as a cleaning agent because it is water soluble and acidic.
- Write an expression for K for this reaction.
- Relate this equilibrium constant to the equilibrium constant for the related reaction:
- The dissolution of Na2SO4 in water produces the equilibrium reaction with K = 8.33 × 10−13. What is the concentration of OH− in a solution formed from the dissolution of 1.00 g of sodium sulfate to make 150.0 mL of aqueous solution? Neglect the autoionization of water in your answer.
-
♦ One of the Venera orbiter satellites measured S2 concentrations at the surface of Venus. The resulting thermochemical data suggest that S2 formation at the planet’s surface occurs via the following equilibrium reaction: Write an expression for K for this reaction and then relate this expression to those for the following reactions:
- At 450°C, the equilibrium pressure of CO2 is 85.0 atm, SO2 is 1.0 atm, CO is 1.0 atm, and S2 is 3.0 × 10−8 atm. What are K and Kp at this temperature? What is the concentration of S2?
-
♦ Until the early part of the 20th century, commercial production of sulfuric acid was carried out by the “lead-chamber” process, in which SO2 was oxidized to H2SO4 in a lead-lined room. This process may be summarized by the following sequence of reactions:
- Write the equilibrium constant expressions for reactions 1 and 2 and the sum of the reactions (reaction 3).
- Show that K3 = K1 × K2.
- If insufficient water is added in reaction 2 such that the reaction becomes does K3 still equal K1 × K2?
- Based on part c, write the equilibrium constant expression for K2.
-
Phosgene (carbonic dichloride, COCl2) is a colorless, highly toxic gas with an odor similar to that of moldy hay. Used as a lethal gas in war, phosgene can be immediately fatal; inhalation can cause either pneumonia or pulmonary edema. For the equilibrium reaction Kp is 0.680 at −10°C. If the initial pressure of COCl2 is 0.681 atm, what is the partial pressure of each component of this equilibrium system? Is the formation of products or reactant favored in this reaction?
-
♦ British bituminous coal has a high sulfur content and produces much smoke when burned. In 1952, burning of this coal in London led to elevated levels of smog containing high concentrations of sulfur dioxide, a lung irritant, and more than 4000 people died. Sulfur dioxide emissions can be converted to SO3 and ultimately to H2SO4, which is the cause of acid rain. The initial reaction is for which Kp = 44.
- Given this Kp, are product or reactants favored in this reaction?
- What is the partial pressure of each species under equilibrium conditions if the initial pressure of each reactant is 0.50 atm?
- Would an increase in pressure favor the formation of product or reactants? Why?
-
Oxyhemoglobin is the oxygenated form of hemoglobin, the oxygen-carrying pigment of red blood cells. Hemoglobin is built from α and β protein chains. Assembly of the oxygenated (oxy) and deoxygenated (deoxy) β-chains has been studied with the following results:
Is it more likely that hemoglobin β chains assemble in an oxygenated or deoxygenated state? Explain your answer.
-
♦ Inorganic weathering reactions can turn silicate rocks, such as diopside (CaMgSi2O6), to carbonate via the following reaction:
Write an expression for the equilibrium constant. Although this reaction occurs on both Earth and Venus, the high surface temperature of Venus causes the reaction to be driven in one direction on that planet. Predict whether high temperatures will drive the reaction to the right or the left and then justify your answer. The estimated partial pressure of carbon dioxide on Venus is 85 atm due to the dense Venusian atmosphere. How does this pressure influence the reaction?
-
Silicon and its inorganic compounds are widely used to manufacture textile glass fibers, cement, ceramic products, and synthetic fillers. Two of the most important industrially utilized silicon halides are SiCl4 and SiHCl3, formed by reaction of elemental silicon with HCl at temperatures greater than 300°C:
Which of these two reactions is favored by increasing [HCl]? by decreasing the volume of the system?
-
♦ The first step in the utilization of glucose in humans is the conversion of glucose to glucose-6-phosphate via the transfer of a phosphate group from ATP (adenosine triphosphate), which produces glucose-6-phosphate and ADP (adenosine diphosphate):
- Is the formation of products or reactants favored in this reaction?
- Would K increase, decrease, or remain the same if the glucose concentration were doubled?
- If −RT ln K = −RT′ ln K', what would K be if the temperature were decreased to 0°C?
- Is the formation of products favored by an increase or a decrease in the temperature of the system?
-
In the presence of O2, the carbon atoms of glucose can be fully oxidized to CO2 with a release of free energy almost 20 times greater than that possible under conditions in which O2 is not present. In many animal cells, the TCA cycle (tricarboxylic acid cycle) is the second stage in the complete oxidation of glucose. One reaction in the TCA cycle is the conversion of citrate to isocitrate, for which K = 0.08 in the forward direction. Speculate why the cycle continues despite this unfavorable value of K. What happens if the citrate concentration increases?
-
♦ Soil is an open system, subject to natural inputs and outputs that may change its chemical composition. Aqueous-phase, adsorbed, and solid-phase forms of Al(III) are of critical importance in controlling the acidity of soils, although industrial effluents, such as sulfur and nitrogen oxide gases, and fertilizers containing nitrogen can also have a large effect. Dissolution of the mineral gibbsite, which contains Al3+ in the form Al(OH)3(s), occurs in soil according to the following reaction:
When gibbsite is in a highly crystalline state, K = 9.35 for this reaction at 298 K. In the microcrystalline state, K = 8.11. Is this change consistent with the increased surface area of the microcrystalline state?
Problems marked with a ♦ involve multiple concepts.
Answers
-
-
-
- reactant
- [Cl2] = [PCl3] = 0.0836 M; [PCl5] = 0.166 M
- increasing pressure favors reactant (PCl5)
- 1.59 × 103 kg; 52.5%
-
-
Products are favored; high concentrations of water will favor chloral hydrate formation.
-
-
-
-
- Kp = 1.6; K = 93; [S2] = 5.1 × 10−10 M
-
-
= 0.421 atm; = 0.260 atm; reactants are slightly favored.
-
-
-
-
Both reactions are favored by increasing [HCl] and decreasing volume.
-
-
-




