This is “Aqueous Solutions”, section 4.1 from the book Principles of General Chemistry (v. 1.0). For details on it (including licensing), click here.
For more information on the source of this book, or why it is available for free, please see the project's home page. You can browse or download additional books there.
4.1 Aqueous Solutions
Learning Objective
- To understand how and why solutions form.
The solvent in aqueous solutions is water, which makes up about 70% of the mass of the human body and is essential for life. Many of the chemical reactions that keep us alive depend on the interaction of water molecules with dissolved compounds. Moreover, as we will discuss in the next chapter, the presence of large amounts of water on Earth’s surface helps maintain its surface temperature in a range suitable for life. In this section, we describe some of the interactions of water with various substances and introduce you to the characteristics of aqueous solutions.
Polar Substances
As shown in Figure 4.1 "The Polar Nature of Water", the individual water molecule consists of two hydrogen atoms bonded to an oxygen atom in a bent (V-shaped) structure. As is typical of group 16 elements, the oxygen atom in each O–H covalent bond attracts electrons more strongly than the hydrogen atom does. Consequently, the oxygen and hydrogen nuclei do not equally share electrons. Instead, hydrogen atoms are electron poor compared with a neutral hydrogen atom and have a partial positive charge, which is indicated by δ+. The oxygen atom, in contrast, is more electron rich than a neutral oxygen atom, so it has a partial negative charge. This charge must be twice as large as the partial positive charge on each hydrogen for the molecule to have a net charge of zero. Thus its charge is indicated by 2δ−. This unequal distribution of charge creates a polar bondA chemical bond in which there is an unequal distribution of charge between the bonding atoms., in which one portion of the molecule carries a partial negative charge, while the other portion carries a partial positive charge (Figure 4.1 "The Polar Nature of Water"). Because of the arrangement of polar bonds in a water molecule, water is described as a polar substance.
Figure 4.1 The Polar Nature of Water
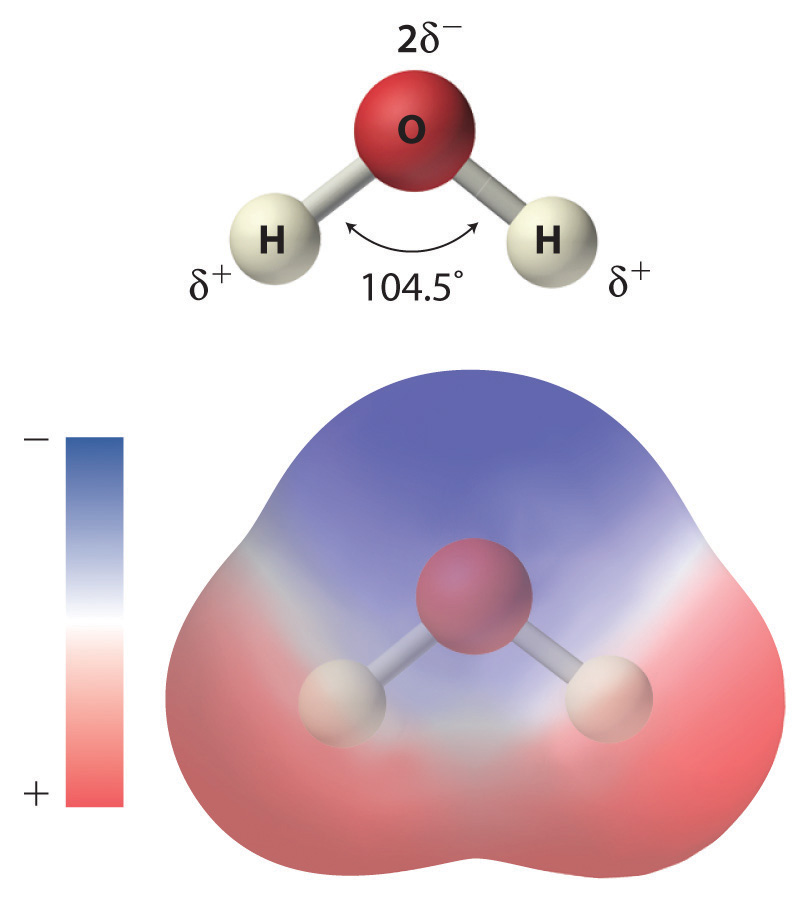
Each water molecule consists of two hydrogen atoms bonded to an oxygen atom in a bent (V-shaped) structure. Because the oxygen atom attracts electrons more strongly than the hydrogen atoms do, the oxygen atom is partially negatively charged (2δ−; blue) and the hydrogen atoms are partially positively charged (δ+; red). For the molecule to have a net charge of zero, the partial negative charge on oxygen must be twice as large as the partial positive charge on each hydrogen.
Because of the asymmetric charge distribution in the water molecule, adjacent water molecules are held together by attractive electrostatic (δ+…δ−) interactions between the partially negatively charged oxygen atom of one molecule and the partially positively charged hydrogen atoms of adjacent molecules (Figure 4.2 "The Structure of Liquid Water"). Energy is needed to overcome these electrostatic attractions. In fact, without them, water would evaporate at a much lower temperature, and neither Earth’s oceans nor we would exist!
Figure 4.2 The Structure of Liquid Water
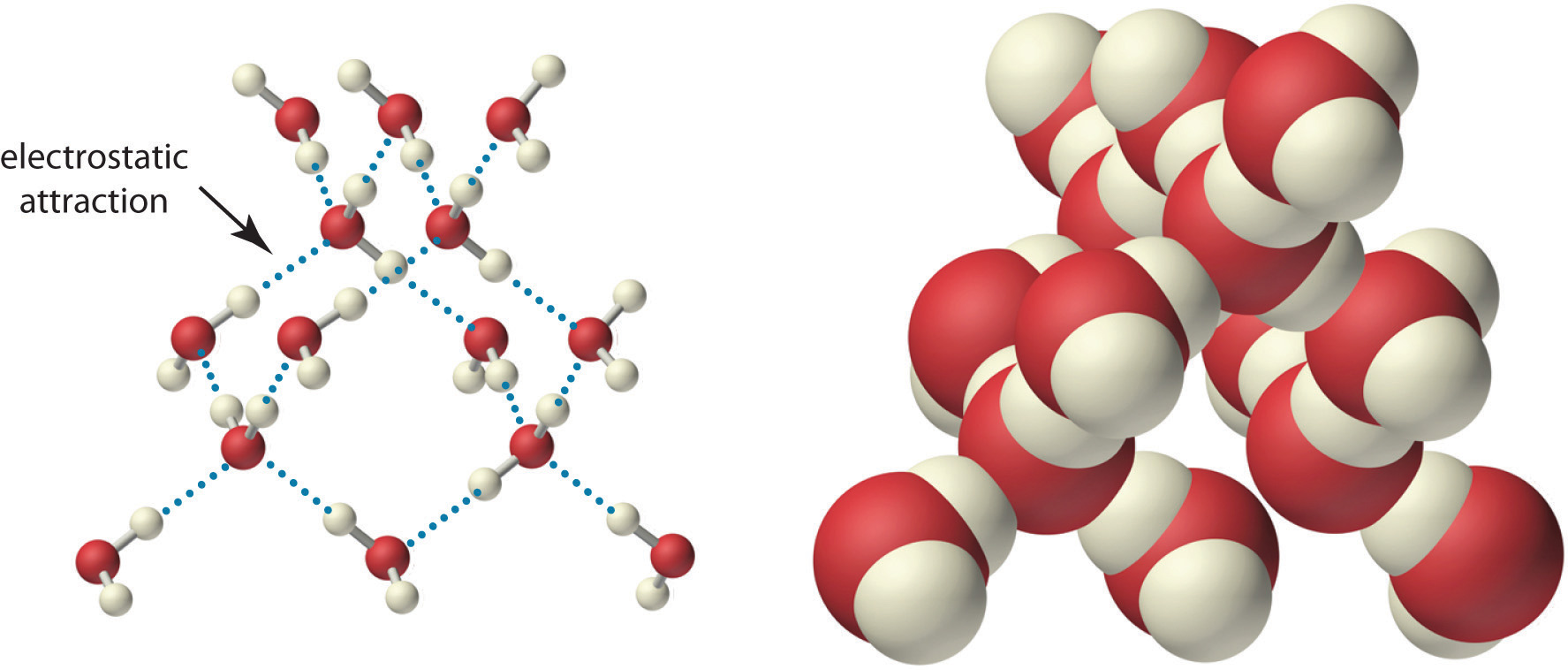
Two views of a water molecule are shown: (a) a ball-and-stick structure and (b) a space-filling model. Water molecules are held together by electrostatic attractions (dotted lines) between the partially negatively charged oxygen atom of one molecule and the partially positively charged hydrogen atoms on adjacent molecules. As a result, the water molecules in liquid water form transient networks with structures similar to that shown. Because the interactions between water molecules are continually breaking and reforming, liquid water does not have a single fixed structure.
Ionic compounds such as sodium chloride (NaCl) are also held together by electrostatic interactions—in this case, between oppositely charged ions in the highly ordered solid, where each ion is surrounded by ions of the opposite charge in a fixed arrangement. In contrast to an ionic solid, the structure of liquid water is not completely ordered because the interactions between molecules in a liquid are constantly breaking and reforming.
The unequal charge distribution in polar liquids such as water makes them good solvents for ionic compounds. When an ionic solid dissolves in water, the ions dissociate. That is, the partially negatively charged oxygen atoms of the H2O molecules surround the cations (Na+ in the case of NaCl), and the partially positively charged hydrogen atoms in H2O surround the anions (Cl−; Figure 4.3 "The Dissolution of Sodium Chloride in Water"). Individual cations and anions that are each surrounded by their own shell of water molecules are called hydrated ionsIndividual cations and anions that are each surrounded by their own shell of water molecules.. We can describe the dissolution of NaCl in water as
Equation 4.1
where (aq) indicates that Na+ and Cl− are hydrated ions.
Figure 4.3 The Dissolution of Sodium Chloride in Water
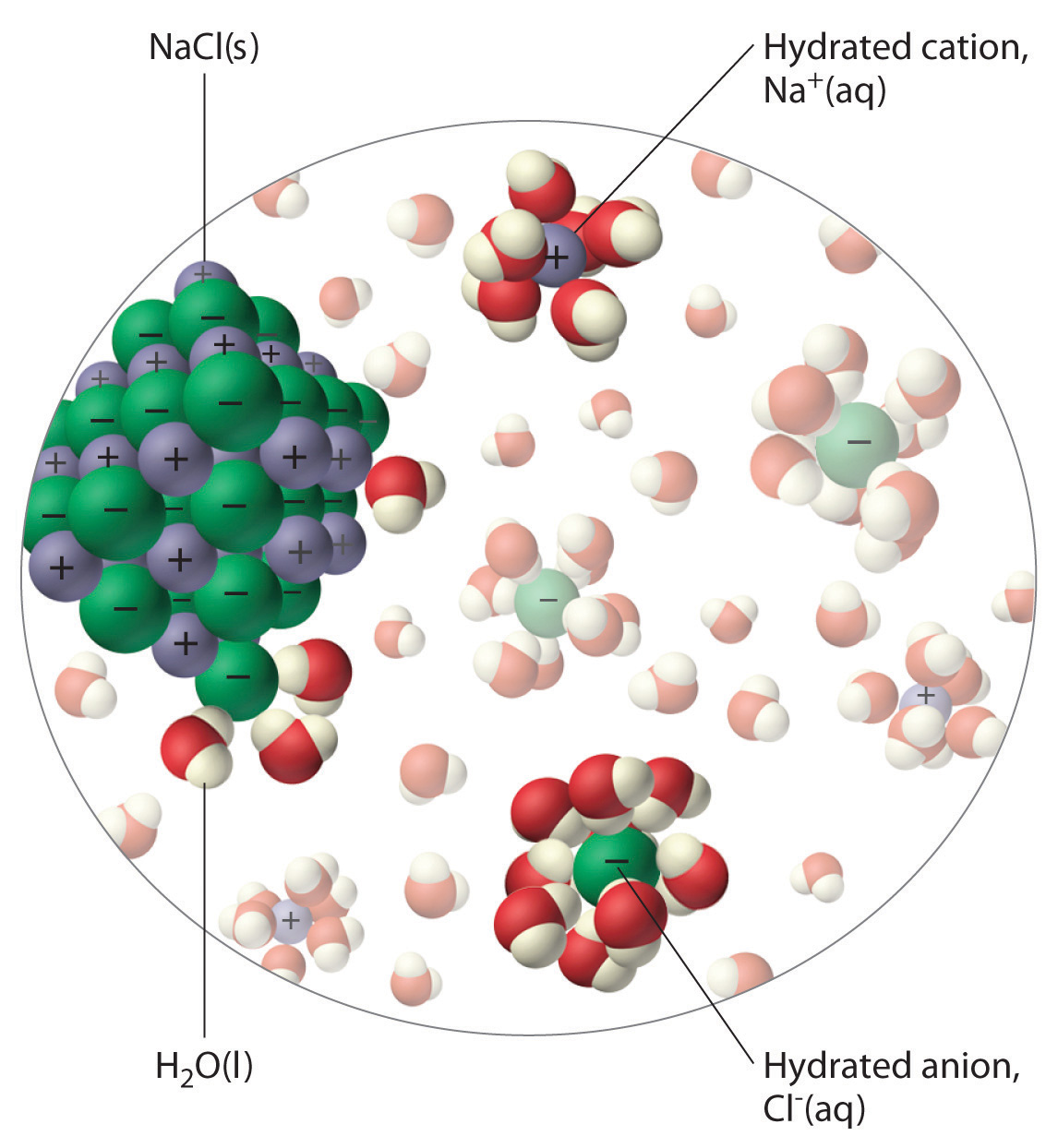
An ionic solid such as sodium chloride dissolves in water because of the electrostatic attraction between the cations (Na+) and the partially negatively charged oxygen atoms of water molecules, and between the anions (Cl−) and the partially positively charged hydrogen atoms of water.
Note the Pattern
Polar liquids are good solvents for ionic compounds.
Electrolytes
When electricity, in the form of an electrical potential, is applied to a solution, ions in solution migrate toward the oppositely charged rod or plate to complete an electrical circuit, whereas neutral molecules in solution do not (Figure 4.4 "The Effect of Ions on the Electrical Conductivity of Water"). Thus solutions that contain ions conduct electricity, while solutions that contain only neutral molecules do not. Electrical current will flow through the circuit shown in Figure 4.4 "The Effect of Ions on the Electrical Conductivity of Water" and the bulb will glow only if ions are present. The lower the concentration of ions in solution, the weaker the current and the dimmer the glow. Pure water, for example, contains only very low concentrations of ions, so it is a poor electrical conductor.
Note the Pattern
Solutions that contain ions conduct electricity.
Figure 4.4 The Effect of Ions on the Electrical Conductivity of Water

An electrical current will flow and light the bulb only if the solution contains ions. (left) Pure water or an aqueous solution of a nonelectrolyte allows almost no current to flow, and the bulb does not light. (right) A strong electrolyte produces many ions, allowing more current to flow and the bulb to shine brightly. Image Credit: Highland Community College, Freeport, IL.
An electrolyteAny compound that can form ions when dissolved in water (c.f. nonelectrolytes). Electrolytes may be strong or weak. is any compound that can form ions when it dissolves in water. When strong electrolytesAn electrolyte that dissociates completely into ions when dissolved in water, thus producing an aqueous solution that conducts electricity very well. dissolve, the constituent ions dissociate completely due to strong electrostatic interactions with the solvent, producing aqueous solutions that conduct electricity very well (Figure 4.4 "The Effect of Ions on the Electrical Conductivity of Water"). Examples include ionic compounds such as barium chloride (BaCl2) and sodium hydroxide (NaOH), which are both strong electrolytes and dissociate as follows:
Equation 4.2
Equation 4.3
The single arrows from reactant to products in Equation 4.2 and Equation 4.3 indicate that dissociation is complete.
When weak electrolytesA compound that produces relatively few ions when dissolved in water, thus producing an aqueous solution that conducts electricity poorly. dissolve, they produce relatively few ions in solution. This does not mean that the compounds do not dissolve readily in water; many weak electrolytes contain polar bonds and are therefore very soluble in a polar solvent such as water. They do not completely dissociate to form ions, however, because of their weaker electrostatic interactions with the solvent. Because very few of the dissolved particles are ions, aqueous solutions of weak electrolytes do not conduct electricity as well as solutions of strong electrolytes. One such compound is acetic acid (CH3CO2H), which contains the –CO2H unit. Although it is soluble in water, it is a weak acid and therefore also a weak electrolyte. Similarly, ammonia (NH3) is a weak base and therefore a weak electrolyte. The behavior of weak acids and weak bases will be described in more detail when we discuss acid–base reactions later in this chapter.
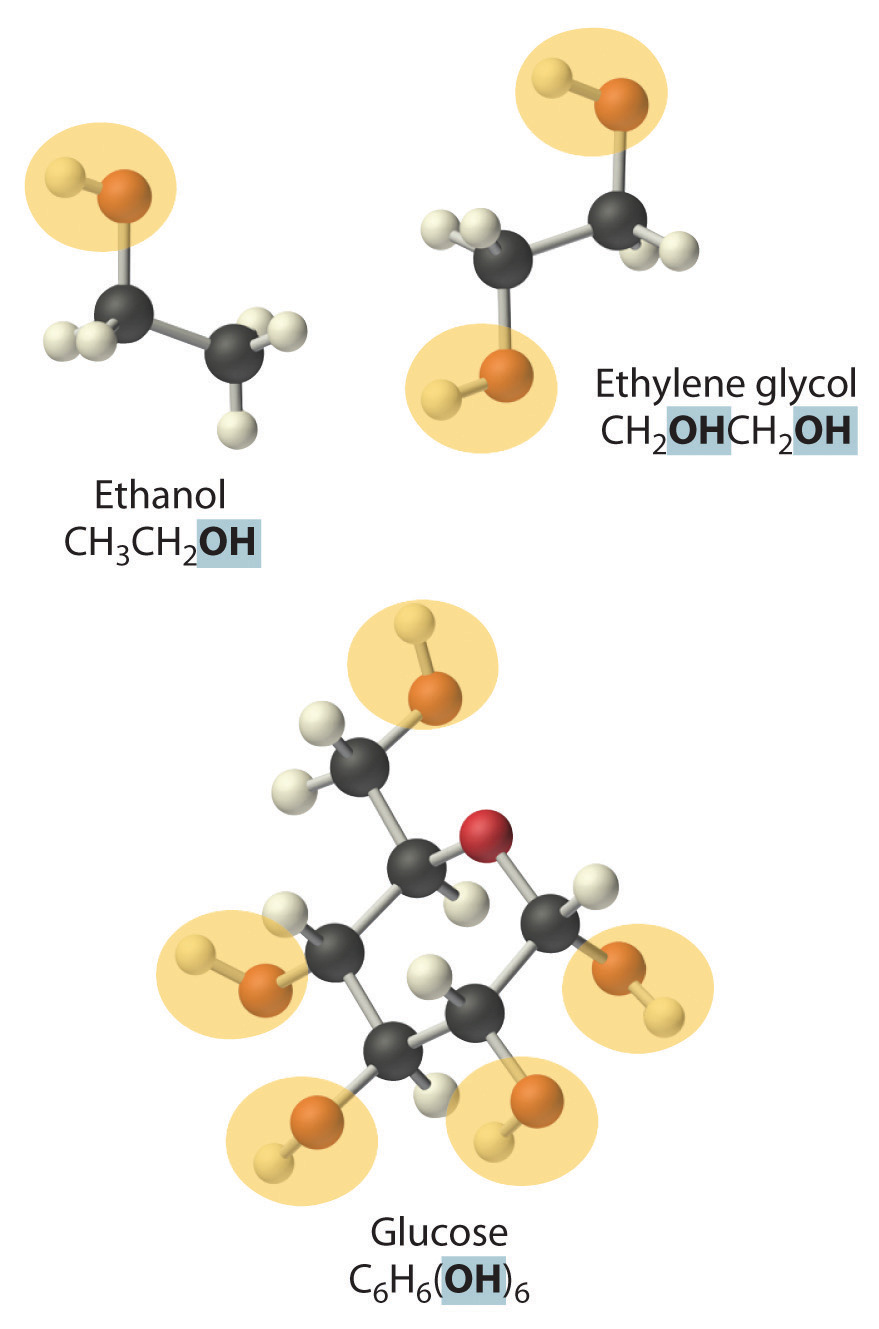
NonelectrolytesA substance that dissolves in water to form neutral molecules and has essentially no effect on electrical conductivity. that dissolve in water do so as neutral molecules and thus have essentially no effect on conductivity. Examples of nonelectrolytes that are very soluble in water but that are essentially nonconductive are ethanol, ethylene glycol, glucose, and sucrose, all of which contain the –OH group that is characteristic of alcohols. In futue chapters, we will discuss why alcohols and carboxylic acids behave differently in aqueous solution; for now, however, you can simply look for the presence of the –OH and –CO2H groups when trying to predict whether a substance is a strong electrolyte, a weak electrolyte, or a nonelectrolyte. In addition to alcohols, two other classes of organic compounds that are nonelectrolytes are aldehydesA class of organic compounds that has the general form RCHO, in which the carbon atom of the carbonyl group is bonded to a hydrogen atom and an R group. The R group may be either another hydrogen atom or an alkyl group (c.f. ketone). and ketonesA class of organic compounds with the general form RC(O)R’, in which the carbon atom of the carbonyl group is bonded to two alkyl groups (c.f. aldehyde). The alkyl groups may be the same or different., whose general structures are shown here. The distinctions between soluble and insoluble substances and between strong, weak, and nonelectrolytes are illustrated in Figure 4.5 "The Difference between Soluble and Insoluble Compounds (a) and Strong, Weak, and Nonelectrolytes (b)".
Note the Pattern
Ionic substances and carboxylic acids are electrolytes; alcohols, aldehydes, and ketones are nonelectrolytes.
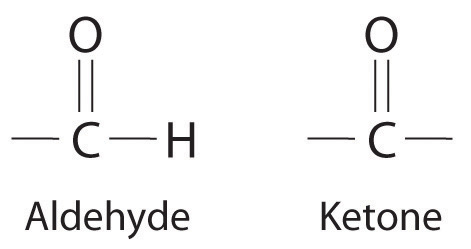
General structure of an aldehyde and a ketone. Notice that both contain the C=O group.
Figure 4.5 The Difference between Soluble and Insoluble Compounds (a) and Strong, Weak, and Nonelectrolytes (b)
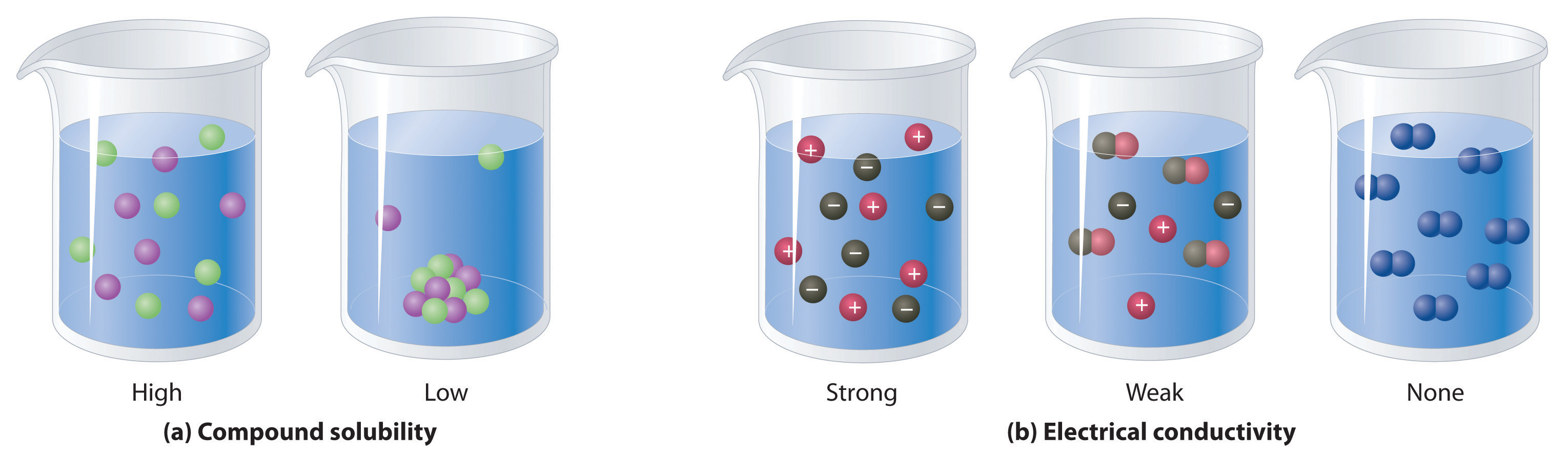
When a soluble compound dissolves, its constituent atoms, molecules, or ions disperse throughout the solvent. In contrast, the constituents of an insoluble compound remain associated with one another in the solid. A soluble compound is a strong electrolyte if it dissociates completely into ions, a weak electrolyte if it dissociates only slightly into ions, and a nonelectrolyte if it dissolves to produce only neutral molecules.
Example 1
Predict whether each compound is a strong electrolyte, a weak electrolyte, or a nonelectrolyte in water.
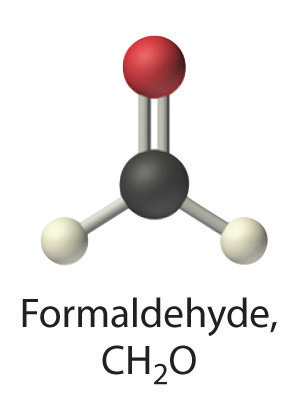
formaldehyde
- cesium chloride
Given: compound
Asked for: relative ability to form ions in water
Strategy:
A Classify the compound as ionic or covalent.
B If the compound is ionic and dissolves, it is a strong electrolyte that will dissociate in water completely to produce a solution that conducts electricity well. If the compound is covalent and organic, determine whether it contains the carboxylic acid group. If the compound contains this group, it is a weak electrolyte. If not, it is a nonelectrolyte.
Solution:
- A Formaldehyde is an organic compound, so it is covalent. B It contains an aldehyde group, not a carboxylic acid group, so it should be a nonelectrolyte.
- A Cesium chloride (CsCl) is an ionic compound that consists of Cs+ and Cl− ions. B Like virtually all other ionic compounds that are soluble in water, cesium chloride will dissociate completely into Cs+(aq) and Cl−(aq) ions. Hence it should be a strong electrolyte.
Exercise
Predict whether each compound is a strong electrolyte, a weak electrolyte, or a nonelectrolyte in water.
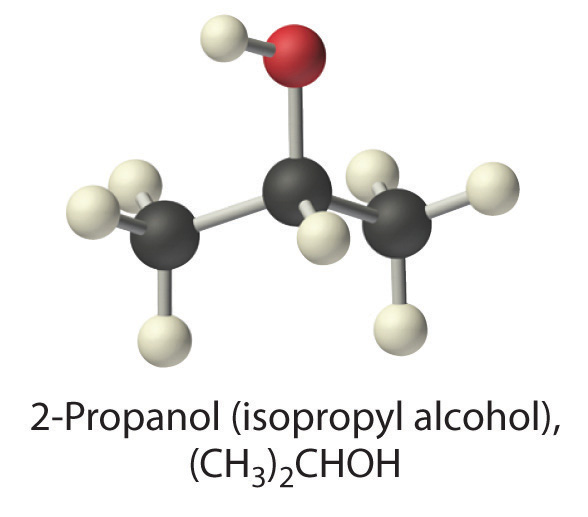
(CH3)2CHOH (2-propanol)
- ammonium sulfate
Answer:
- nonelectrolyte
- strong electrolyte
Summary
Most chemical reactions are carried out in solutions, which are homogeneous mixtures of two or more substances. In a solution, a solute (the substance present in the lesser amount) is dispersed in a solvent (the substance present in the greater amount). Aqueous solutions contain water as the solvent, whereas nonaqueous solutions have solvents other than water.
Polar substances, such as water, contain asymmetric arrangements of polar bonds, in which electrons are shared unequally between bonded atoms. Polar substances and ionic compounds tend to be most soluble in water because they interact favorably with its structure. In aqueous solution, dissolved ions become hydrated; that is, a shell of water molecules surrounds them.
Substances that dissolve in water can be categorized according to whether the resulting aqueous solutions conduct electricity. Strong electrolytes dissociate completely into ions to produce solutions that conduct electricity well. Weak electrolytes produce a relatively small number of ions, resulting in solutions that conduct electricity poorly. Nonelectrolytes dissolve as uncharged molecules and have no effect on the electrical conductivity of water.
Key Takeaway
- Aqueous solutions can be classified as polar or nonpolar depending on how well they conduct electricity.
Conceptual Problems
-
What are the advantages to carrying out a reaction in solution rather than simply mixing the pure reactants?
-
What types of compounds dissolve in polar solvents?
-
Describe the charge distribution in liquid water. How does this distribution affect its physical properties?
-
Must a molecule have an asymmetric charge distribution to be polar? Explain your answer.
-
Why are many ionic substances soluble in water?
-
Explain the phrase like dissolves like.
-
What kinds of covalent compounds are soluble in water?
-
Why do most aromatic hydrocarbons have only limited solubility in water? Would you expect their solubility to be higher, lower, or the same in ethanol compared with water? Why?
-
Predict whether each compound will dissolve in water and explain why.
- toluene
- acetic acid
- sodium acetate
- butanol
- pentanoic acid
-
Predict whether each compound will dissolve in water and explain why.
- ammonium chloride
- 2-propanol
- heptane
- potassium dichromate
- 2-octanol
-
Given water and toluene, predict which is the better solvent for each compound and explain your reasoning.
- sodium cyanide
- benzene
- acetic acid
- sodium ethoxide (CH3CH2ONa)
-
Of water and toluene, predict which is the better solvent for each compound and explain your reasoning.
- t-butanol
- calcium chloride
- sucrose
- cyclohexene
-
Compound A is divided into three equal samples. The first sample does not dissolve in water, the second sample dissolves only slightly in ethanol, and the third sample dissolves completely in toluene. What does this suggest about the polarity of A?
-
You are given a mixture of three solid compounds—A, B, and C—and are told that A is a polar compound, B is slightly polar, and C is nonpolar. Suggest a method for separating these three compounds.
-
A laboratory technician is given a sample that contains only sodium chloride, sucrose, and cyclodecanone (a ketone). You must tell the technician how to separate these three compounds from the mixture. What would you suggest?
-
Many over-the-counter drugs are sold as ethanol/water solutions rather than as purely aqueous solutions. Give a plausible reason for this practice.
-
What distinguishes a weak electrolyte from a strong electrolyte?
-
Which organic groups result in aqueous solutions that conduct electricity?
-
It is considered highly dangerous to splash barefoot in puddles during a lightning storm. Why?
-
Which solution(s) would you expect to conduct electricity well? Explain your reasoning.
- an aqueous solution of sodium chloride
- a solution of ethanol in water
- a solution of calcium chloride in water
- a solution of sucrose in water
-
Which solution(s) would you expect to conduct electricity well? Explain your reasoning.
- an aqueous solution of acetic acid
- an aqueous solution of potassium hydroxide
- a solution of ethylene glycol in water
- a solution of ammonium chloride in water
-
Which of the following is a strong electrolyte, a weak electrolyte, or a nonelectrolyte in an aqueous solution? Explain your reasoning.
- potassium hydroxide
- ammonia
- calcium chloride
- butanoic acid
-
Which of the following is a strong electrolyte, a weak electrolyte, or a nonelectrolyte in an aqueous solution? Explain your reasoning.
- magnesium hydroxide
- butanol
- ammonium bromide
- pentanoic acid
-
Which of the following is a strong electrolyte, a weak electrolyte, or a nonelectrolyte in aqueous solution? Explain your reasoning.
- H2SO4
- diethylamine
- 2-propanol
- ammonium chloride
- propanoic acid
Answers
-
-
-
-
-
Ionic compounds such as NaCl are held together by electrostatic interactions between oppositely charged ions in the highly ordered solid. When an ionic compound dissolves in water, the partially negatively charged oxygen atoms of the H2O molecules surround the cations, and the partially positively charged hydrogen atoms in H2O surround the anions. The favorable electrostatic interactions between water and the ions compensate for the loss of the electrostatic interactions between ions in the solid.
-
-
-
-
- Because toluene is an aromatic hydrocarbon that lacks polar groups, it is unlikely to form a homogenous solution in water.
- Acetic acid contains a carboxylic acid group attached to a small alkyl group (a methyl group). Consequently, the polar characteristics of the carboxylic acid group will be dominant, and acetic acid will form a homogenous solution with water.
- Because most sodium salts are soluble, sodium acetate should form a homogenous solution with water.
- Like all alcohols, butanol contains an −OH group that can interact well with water. The alkyl group is rather large, consisting of a 4-carbon chain. In this case, the nonpolar character of the alkyl group is likely to be as important as the polar character of the –OH, decreasing the likelihood that butanol will form a homogeneous solution with water.
- Like acetic acid, pentanoic acid is a carboxylic acid. Unlike acetic acid, however, the alkyl group is rather large, consisting of a 4-carbon chain as in butanol. As with butanol, the nonpolar character of the alkyl group is likely to be as important as the polar character of the carboxylic acid group, making it unlikely that pentanoic acid will form a homogeneous solution with water. (In fact, the solubility of both butanol and pentanoic acid in water is quite low, only about 3 g per 100 g water at 25°C.)
-
-
-
-
-
-
-
-
An electrolyte is any compound that can form ions when it dissolves in water. When a strong electrolyte dissolves in water, it dissociates completely to give the constituent ions. In contrast, when a weak electrolyte dissolves in water, it produces relatively few ions in solution.
-
-
-
-
-
-
-




