This is "Unit 9", section 9.2 from the book General Chemistry (v. 1.0).
9.2 Molar Mass
Learning Objective
- To calculate the molecular mass of a covalent compound and the formula mass of an ionic compound and to calculate the number of atoms, molecules, or formula units in a sample of a substance.
As you learned in Chapter 1 "Introduction to Chemistry", the mass number is the sum of the numbers of protons and neutrons present in the nucleus of an atom. The mass number is an integer that is approximately equal to the numerical value of the atomic mass. Although the mass number is unitless, it is assigned units called atomic mass units (amu). Because a molecule or a polyatomic ion is an assembly of atoms whose identities are given in its molecular or ionic formula, we can calculate the average atomic mass of any molecule or polyatomic ion from its composition by adding together the masses of the constituent atoms. The average mass of a monatomic ion is the same as the average mass of an atom of the element because the mass of electrons is so small that it is insignificant in most calculations.
Molecular and Formula Masses
The molecular massThe sum of the average masses of the atoms in one molecule of a substance, each multiplied by its subscript. of a substance is the sum of the average masses of the atoms in one molecule of a substance. It is calculated by adding together the atomic masses of the elements in the substance, each multiplied by its subscript (written or implied) in the molecular formula. Because the units of atomic mass are atomic mass units, the units of molecular mass are also atomic mass units. The procedure for calculating molecular masses is illustrated in Example 9.2-1.
Example 9.2-1
Calculate the molecular mass of ethanol, whose condensed structural formula is CH3CH2OH. Among its many uses, ethanol is a fuel for internal combustion engines.
Given: molecule
Asked for: molecular mass
Strategy:
A Determine the number of atoms of each element in the molecule.
B Obtain the atomic masses of each element from the periodic table and multiply the atomic mass of each element by the number of atoms of that element.
C Add together the masses to give the molecular mass.
Solution:
A The molecular formula of ethanol may be written in three different ways: CH3CH2OH (which illustrates the presence of an ethyl group, CH3CH2−, and an −OH group), C2H5OH, and C2H6O; all show that ethanol has two carbon atoms, six hydrogen atoms, and one oxygen atom.
B Taking the atomic masses from the periodic table, we obtain
C Adding together the masses gives the molecular mass:
24.022 amu + 6.0474 amu + 15.9994 amu = 46.069 amuAlternatively, we could have used unit conversions to reach the result in one step:
The same calculation can also be done in a tabular format, which is especially helpful for more complex molecules:
Exercise
Calculate the molecular mass of trichlorofluoromethane, also known as Freon-11, whose condensed structural formula is CCl3F. Until recently, it was used as a refrigerant. The structure of a molecule of Freon-11 is as follows:
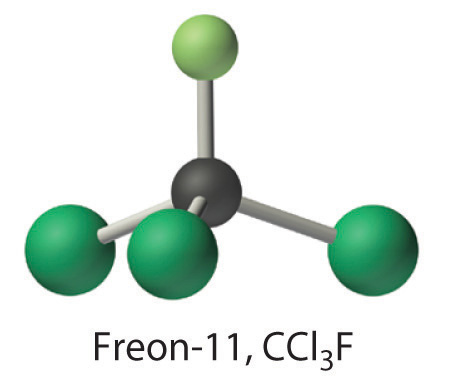
Answer: 137.368 amu
Unlike molecules, which have covalent bonds, ionic compounds do not have a readily identifiable molecular unit. So for ionic compounds we use the formula mass (also called the empirical formula massAnother name for formula mass.) of the compound rather than the molecular mass. The formula massThe sum of the atomic masses of all the elements in the empirical formula, each multiplied by its subscript. is the sum of the atomic masses of all the elements in the empirical formula, each multiplied by its subscript (written or implied). It is directly analogous to the molecular mass of a covalent compound. Once again, the units are atomic mass units.
Note the Pattern
Atomic mass, molecular mass, and formula mass all have the same units: atomic mass units.
Example 9.2-2
Calculate the formula mass of Ca3(PO4)2, commonly called calcium phosphate. This compound is the principal source of calcium found in bovine milk.
Given: ionic compound
Asked for: formula mass
Strategy:
A Determine the number of atoms of each element in the empirical formula.
B Obtain the atomic masses of each element from the periodic table and multiply the atomic mass of each element by the number of atoms of that element.
C Add together the masses to give the formula mass.
Solution:
A The empirical formula—Ca3(PO4)2—indicates that the simplest electrically neutral unit of calcium phosphate contains three Ca2+ ions and two PO43− ions. The formula mass of this molecular unit is calculated by adding together the atomic masses of three calcium atoms, two phosphorus atoms, and eight oxygen atoms.
B Taking atomic masses from the periodic table, we obtain
C Adding together the masses gives the formula mass of Ca3(PO4)2:
120.234 amu + 61.947522 amu + 127.9952 amu = 310.177 amuWe could also find the formula mass of Ca3(PO4)2 in one step by using unit conversions or a tabular format:
Exercise
Calculate the formula mass of Si3N4, commonly called silicon nitride. It is an extremely hard and inert material that is used to make cutting tools for machining hard metal alloys.
Answer: 140.29 amu
Molar Mass
The concept of the mole allows us to count a specific number of individual atoms and molecules by weighing measurable quantities of elements and compounds. To obtain 1 mol of carbon-12 atoms, we would weigh out 12 g of isotopically pure carbon-12. Because each element has a different atomic mass, however, a mole of each element has a different mass, even though it contains the same number of atoms (6.022 × 1023). This is analogous to the fact that a dozen extra large eggs weighs more than a dozen small eggs, or that the total weight of 50 adult humans is greater than the total weight of 50 children. Because of the way in which the mole is defined, for every element the number of grams in a mole is the same as the number of atomic mass units in the atomic mass of the element. For example, the mass of 1 mol of magnesium (atomic mass = 24.305 amu) is 24.305 g. Because the atomic mass of magnesium (24.305 amu) is slightly more than twice that of a carbon-12 atom (12 amu), the mass of 1 mol of magnesium atoms (24.305 g) is slightly more than twice that of 1 mol of carbon-12 (12 g). Similarly, the mass of 1 mol of helium (atomic mass = 4.002602 amu) is 4.002602 g, which is about one-third that of 1 mol of carbon-12.
The molar massThe mass in grams of 1 mol of a substance. of a substance is defined as the mass in grams of 1 mol of that substance. One mole of isotopically pure carbon-12 has a mass of 12 g. For an element, the molar mass is the mass of 1 mol of atoms of that element; for a covalent molecular compound, it is the mass of 1 mol of molecules of that compound; for an ionic compound, it is the mass of 1 mol of formula units. That is, the molar mass of a substance is the mass (in grams per mole) of 6.022 × 1023 atoms, molecules, or formula units of that substance. In each case, the number of grams in 1 mol is the same as the number of atomic mass units that describe the atomic mass, the molecular mass, or the formula mass, respectively.
Note the Pattern
The molar mass of any substance is its atomic mass, molecular mass, or formula mass in grams per mole.
The periodic table lists the atomic mass of carbon as 12.011 amu; the average molar mass of carbon—the mass of 6.022 × 1023 carbon atoms—is therefore 12.011 g/mol:
| Substance (formula) | Atomic, Molecular, or Formula Mass (amu) | Molar Mass (g/mol) |
|---|---|---|
| carbon (C) | 12.011 (atomic mass) | 12.011 |
| ethanol (C2H5OH) | 46.069 (molecular mass) | 46.069 |
| calcium phosphate [Ca3(PO4)2] | 310.177 (formula mass) | 310.177 |
The molar mass of naturally occurring carbon is different from that of carbon-12 and is not an integer because carbon occurs as a mixture of carbon-12, carbon-13, and carbon-14. One mole of carbon still has 6.022 × 1023 carbon atoms, but 98.89% of those atoms are carbon-12, 1.11% are carbon-13, and a trace (about 1 atom in 1012) are carbon-14. Similarly, the molar mass of uranium is 238.03 g/mol, and the molar mass of iodine is 126.90 g/mol.
The molar mass of ethanol is the mass of ethanol (C2H5OH) that contains 6.022 × 1023 ethanol molecules. As you calculated in Example 1, the molecular mass of ethanol is 46.069 amu. Because 1 mol of ethanol contains 2 mol of carbon atoms (2 × 12.011 g), 6 mol of hydrogen atoms (6 × 1.0079 g), and 1 mol of oxygen atoms (1 × 15.9994 g), its molar mass is 46.069 g/mol. Similarly, the formula mass of calcium phosphate [Ca3(PO4)2] is 310.177 amu, so its molar mass is 310.177 g/mol. This is the mass of calcium phosphate that contains 6.022 × 1023 formula units. Figure 9.2(a) shows samples that contain precisely one molar mass of several common substances.
Figure 9.2(a) Samples of 1 Mol of Some Common Substances

Image Credit: Highland Community College, Freeport, IL
The mole is the basis of quantitative chemistry. It provides chemists with a way to convert easily between the mass of a substance and the number of individual atoms, molecules, or formula units of that substance. Conversely, it enables chemists to calculate the mass of a substance needed to obtain a desired number of atoms, molecules, or formula units. For example, to convert moles of a substance to mass, we use the relationship
Equation 9.2(eq1)
(moles)(molar mass) → massor, more specifically,
Conversely, to convert the mass of a substance to moles, we use
Equation 9.2(eq2)
Be sure to pay attention to the units when converting between mass and moles.
Figure 9.2(b) is a flowchart for converting between mass; the number of moles; and the number of atoms, molecules, or formula units. The use of these conversions is illustrated in Example 9.2-3 and Example 9.2-4.
Figure 9.2(b) A Flowchart for Converting between Mass; the Number of Moles; and the Number of Atoms, Molecules, or Formula Units

Example 9.2-3
For 35.00 g of ethylene glycol (HOCH2CH2OH), which is used in inks for ballpoint pens, calculate the number of
- moles.
- molecules.
Given: mass and molecular formula
Asked for: number of moles and number of molecules
Strategy:
A Use the molecular formula of the compound to calculate its molecular mass in grams per mole.
B Convert from mass to moles by dividing the mass given by the compound’s molar mass.
C Convert from moles to molecules by multiplying the number of moles by Avogadro’s number.
Solution:
A The molecular mass of ethylene glycol can be calculated from its molecular formula using the method illustrated in Example 1:
The molar mass of ethylene glycol is 62.068 g/mol.
B The number of moles of ethylene glycol present in 35.00 g can be calculated by dividing the mass (in grams) by the molar mass (in grams per mole):
So
It is always a good idea to estimate the answer before you do the actual calculation. In this case, the mass given (35.00 g) is less than the molar mass, so the answer should be less than 1 mol. The calculated answer (0.5639 mol) is indeed less than 1 mol, so we have probably not made a major error in the calculations.
C To calculate the number of molecules in the sample, we multiply the number of moles by Avogadro’s number:
Because we are dealing with slightly more than 0.5 mol of ethylene glycol, we expect the number of molecules present to be slightly more than one-half of Avogadro’s number, or slightly more than 3 × 1023 molecules, which is indeed the case.
Exercise
For 75.0 g of CCl3F (Freon-11), calculate the number of
- moles.
- molecules.
Answer:
- 0.546 mol
- 3.29 × 1023 molecules
Example 9.2-4
Calculate the mass of 1.75 mol of each compound.
- S2Cl2 (common name: sulfur monochloride; systematic name: disulfur dichloride)
- Ca(ClO)2 (calcium hypochlorite)
Given: number of moles and molecular or empirical formula
Asked for: mass
Strategy:
A Calculate the molecular mass of the compound in grams from its molecular formula (if covalent) or empirical formula (if ionic).
B Convert from moles to mass by multiplying the moles of the compound given by its molar mass.
Solution:
We begin by calculating the molecular mass of S2Cl2 and the formula mass of Ca(ClO)2.
A The molar mass of S2Cl2 is obtained from its molecular mass as follows:
The molar mass of S2Cl2 is 135.036 g/mol.
B The mass of 1.75 mol of S2Cl2 is calculated as follows:
A The formula mass of Ca(ClO)2 is obtained as follows:
The molar mass of Ca(ClO)2 142.983 g/mol.
B The mass of 1.75 mol of Ca(ClO)2 is calculated as follows:
Because 1.75 mol is less than 2 mol, the final quantity in grams in both cases should be less than twice the molar mass, which it is.
Exercise
Calculate the mass of 0.0122 mol of each compound.
- Si3N4 (silicon nitride), used as bearings and rollers
- (CH3)3N (trimethylamine), a corrosion inhibitor
Answer:
- 1.71 g
- 0.721 g
Example 9.2-5
What is the mass of 3.56 mol of HgCl2? The molar mass of HgCl2 is 271.49 g/mol.
Solution
Use the molar mass as a conversion factor between moles and grams. Because we want to cancel the mole unit and introduce the gram unit, we can use the molar mass as given:
Test Yourself
What is the mass of 33.7 mol of H2O?
Answer
607 g
Example 9.2-6
How many moles of H2O are present in 240.0 g of water (about the mass of a cup of water)?
Solution
Use the molar mass of H2O as a conversion factor from mass to moles. The molar mass of water is (1.0079 + 1.0079 + 15.999) = 18.015 g/mol. However, because we want to cancel the gram unit and introduce moles, we need to take the reciprocal of this quantity, or 1 mol/18.015 g:
Test Yourself
How many moles are present in 35.6 g of H2SO4 (molar mass = 98.08 g/mol)?
Answer
0.363 mol
Other conversion factors, for example, density, can be combined with the definition of mole.
Example 9.2-7
The density of ethanol is 0.789 g/mL. How many moles are in 100.0 mL of ethanol? The molar mass of ethanol is 46.08 g/mol.
Solution
Here, we use density to convert from volume to mass and then use the molar mass to determine the number of moles.
Test Yourself
If the density of benzene, C6H6, is 0.879 g/mL, how many moles are present in 17.9 mL of benzene?
Answer
0.201 mol
Summary
The molecular mass and the formula mass of a compound are obtained by adding together the atomic masses of the atoms present in the molecular formula or empirical formula, respectively; the units of both are atomic mass units (amu). The molar mass of a substance is defined as the mass of 1 mol of that substance, expressed in grams per mole, and is equal to the mass of 6.022 × 1023 atoms, molecules, or formula units of that substance.
Key Takeaway
- To analyze chemical transformations, it is essential to use a standardized unit of measure called the mole.
Numerical Problems
-
Derive an expression that relates the number of molecules in a sample of a substance to its mass and molecular mass.
-
Calculate the molecular mass or formula mass of each compound.
- KCl (potassium chloride)
- NaCN (sodium cyanide)
- H2S (hydrogen sulfide)
- NaN3 (sodium azide)
- H2CO3 (carbonic acid)
- K2O (potassium oxide)
- Al(NO3)3 (aluminum nitrate)
- Cu(ClO4)2 [copper(II) perchlorate]
-
Calculate the molecular mass or formula mass of each compound.
- V2O4 (vanadium(IV) oxide)
- CaSiO3 (calcium silicate)
- BiOCl (bismuth oxychloride)
- CH3COOH (acetic acid)
- Ag2SO4 (silver sulfate)
- Na2CO3 (sodium carbonate)
- (CH3)2CHOH (isopropyl alcohol)
-
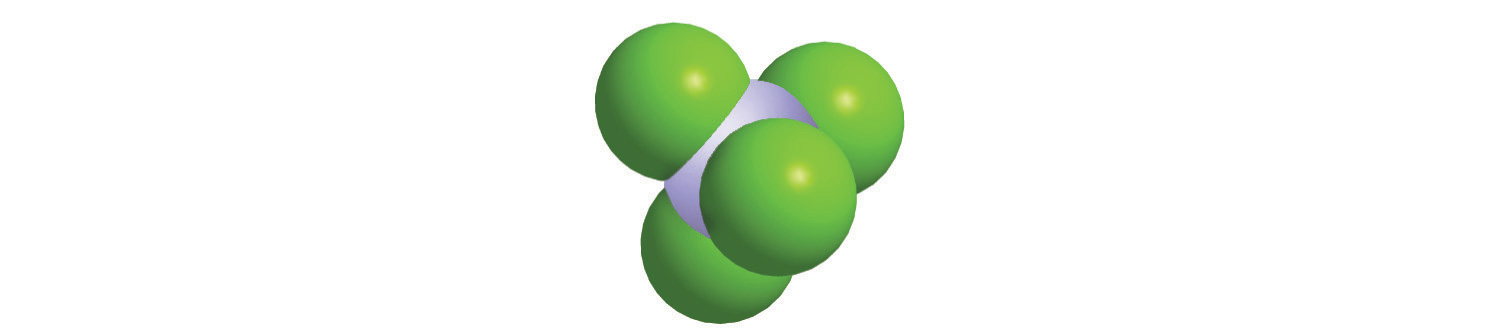
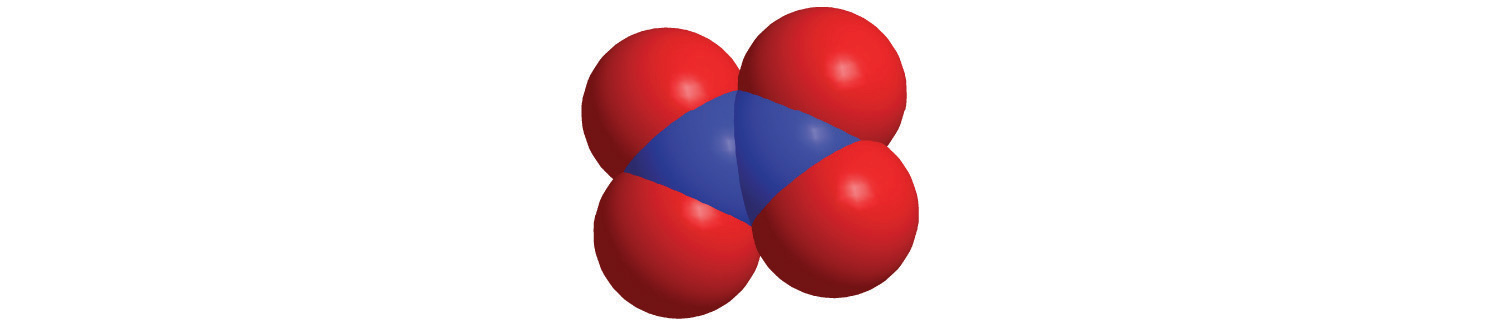
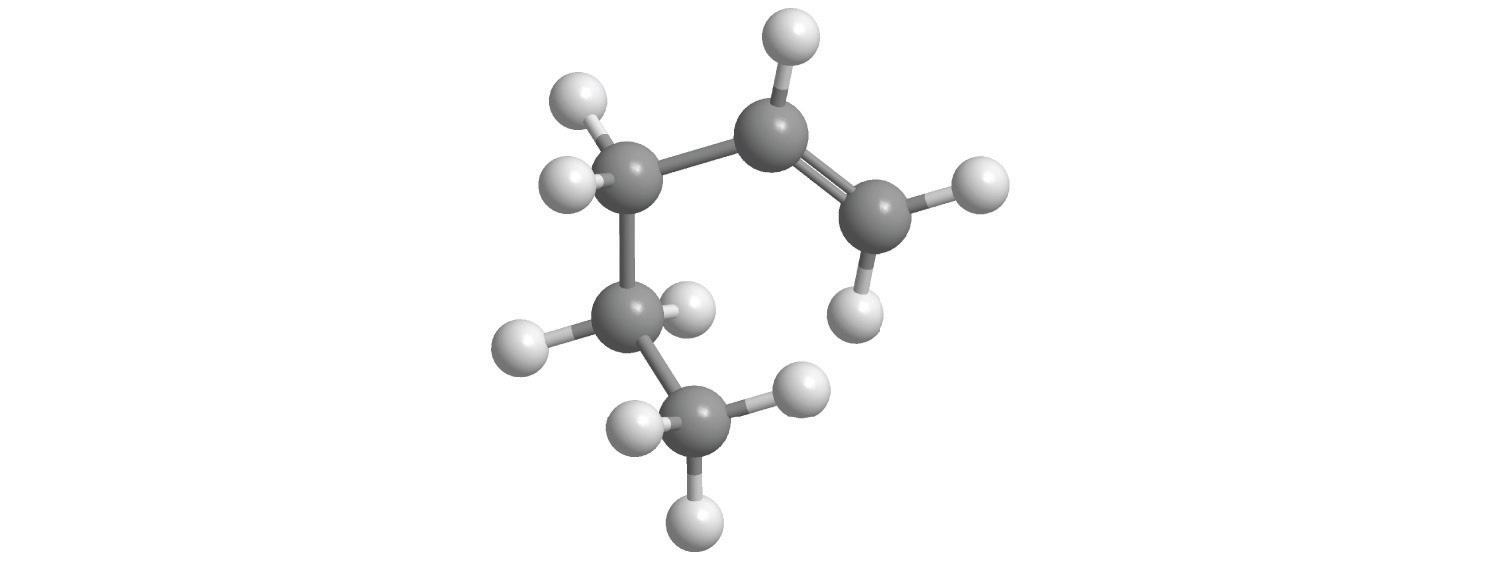
Calculate the molar mass of each compound.
blue = Si, green = Cl
yellow = S, red = O

blue = N, red = O
gray = C, white = H
gray = C, white = H, red = O
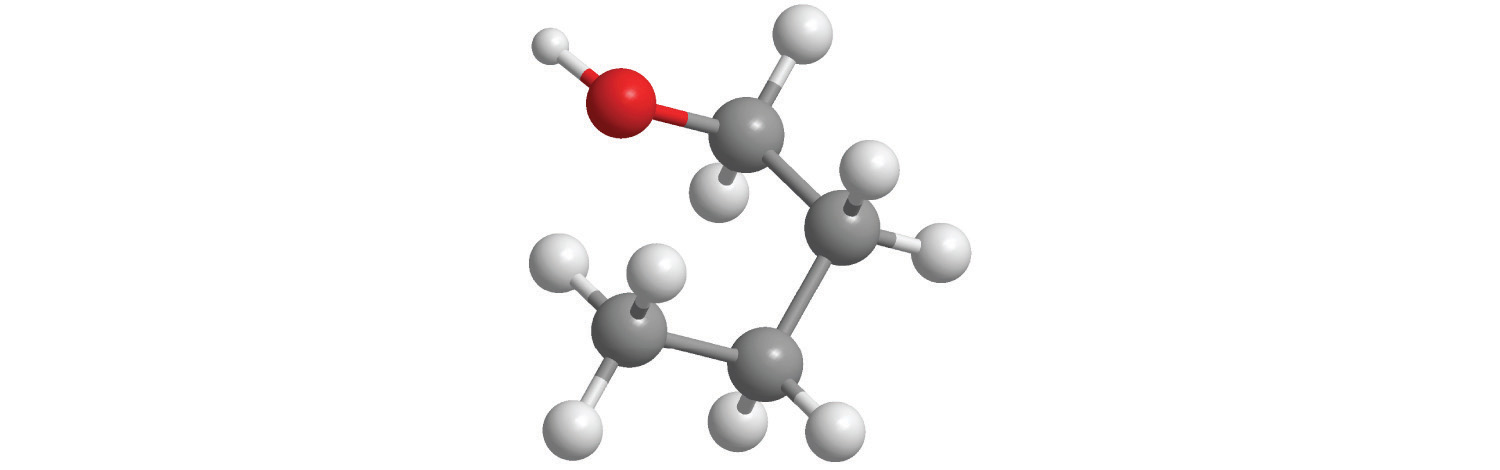
-
Calculate the number of moles in 5.00 × 102 g of each substance. How many molecules or formula units are present in each sample?
- CaO (lime)
- CaCO3 (chalk)
- C12H22O11 [sucrose (cane sugar)]
- NaOCl (bleach)
- CO2 (dry ice)
-
Calculate the mass in grams of each sample.
- 0.520 mol of N2O4
- 1.63 mol of C6H4Br2
- 4.62 mol of (NH4)2SO3
-
Solutions of iodine are used as antiseptics and disinfectants. How many iodine atoms correspond to 11.0 g of molecular iodine (I2)?
-
What is the total number of atoms in each sample?
- 2.48 g of HBr
- 4.77 g of CS2
- 1.89 g of NaOH
- 1.46 g of SrC2O4
-
Decide whether each statement is true or false and explain your reasoning.
- There are more molecules in 0.5 mol of Cl2 than in 0.5 mol of H2.
- One mole of H2 has 6.022 × 1023 hydrogen atoms.
- The molecular mass of H2O is 18.0 amu.
- The formula mass of benzene (C6H12) is 78 amu.
-
Complete the following table.
Substance Mass (g) Number of Moles Number of Molecules or Formula Units Number of Atoms or Ions MgCl2 37.62 AgNO3 2.84 BH4Cl 8.93 × 1025 K2S 7.69 × 1026 H2SO4 1.29 C6H14 11.84 HClO3 2.45 × 1026 -
Give the formula mass or the molecular mass of each substance.
- PbClF
- Cu2P2O7
- BiONO3
- Tl2SeO4
-
Give the formula mass or the molecular mass of each substance.
- MoCl5
- B2O3
- UO2CO3
- NH4UO2AsO4
-
Determine the molar mass of each substance.
- Si
- SiH4
- K2O
-
Determine the molar mass of each substance.
- Cl2
- SeCl2
- Ca(C2H3O2)2
-
Determine the molar mass of each substance.
- Al
- Al2O3
- CoCl3
-
Determine the molar mass of each substance.
- O3
- NaI
- C12H22O11
-
What is the mass of 4.44 mol of Rb?
-
What is the mass of 0.311 mol of Xe?
-
What is the mass of 12.34 mol of Al2(SO4)3?
-
What is the mass of 0.0656 mol of PbCl2?
-
How many moles are present in 45.6 g of CO?
-
How many moles are present in 0.00339 g of LiF?
-
How many moles are present in 1.223 g of SF6?
-
How many moles are present in 48.8 g of BaCO3?
-
How many moles are present in 54.8 mL of mercury if the density of mercury is 13.6 g/mL?
-
How many moles are present in 56.83 mL of O2 if the density of O2 is 0.00133 g/mL?
Answers
-
(mass/molar mass) × 6.02 × 1023= number of molecules
-
-
- 165.9 amu
- 116.2 amu
- 260.4 amu
- 60.03 amu
- 311.8 amu
- 106.0 amu
- 60.03 amu
-
-
- 8.92 mol, 5.37 × 1024 formula units
- 5.00 mol, 3.01 × 1024 formula units
- 1.46 mol, 8.80 × 1023 molecules
- 6.71 mol, 4.04 × 1024 formula units
- 11.4 mol, 6.84 × 1024 molecules
-
-
5.22 × 1022 atoms
-
-
- False, see the answer to question 5 in the numerical exercises for section 9.1.
- False, see the answer to question 5 in the numerical exercises for section 9.1.
- True, H2O is a molecular substance. Each H contributes 1 amu to the mass and oxygen contributes 16 amu to the mass.
- True, though formula mass is often used for salts, and perhaps molecular mass would be more appropriate, benzene certainly has a formula, C6H12, and can therefore have a formula mass. Each carbon contributes 12 amu and each hydrogen contributes 1 amu.
-
-
- 261.7 amu
- 301 amu
- 287 amu
- 551.8 amu
-
-
- 28.086 g
- 32.118 g
- 94.195 g
-
-
- 26.981 g
- 101.959 g
- 165.292 g
-
-
379 g
-
-
4222 g
-
-
1.63 mol
-
-
0.008374 mol
-
-
3.72 mol
-





