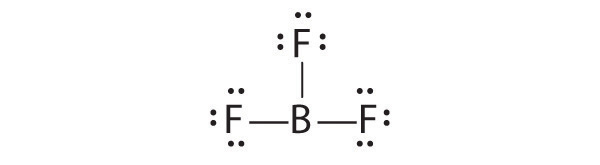
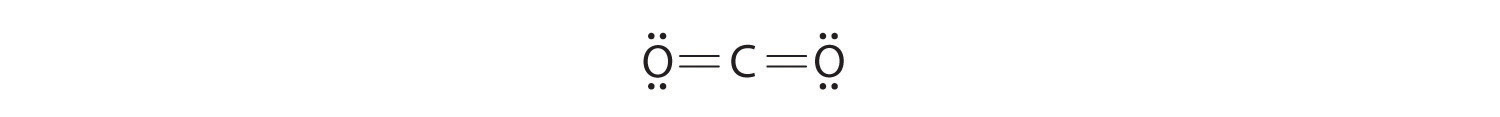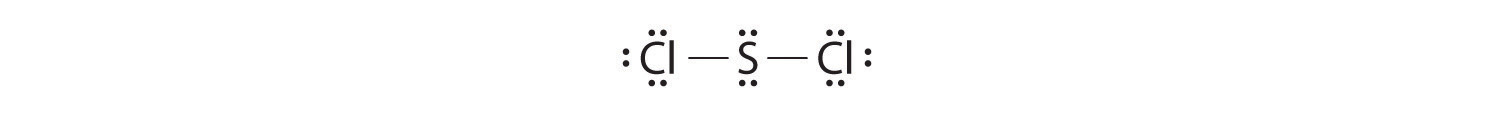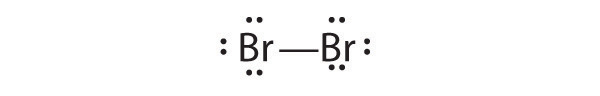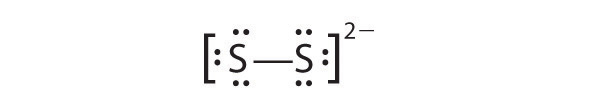This is "Unit 6", section 6.1 from the book General Chemistry (v. 1.0).
6.1 Lewis Structures and Covalent Bonding
Learning Objectives
- To use Lewis dot symbols to explain the stoichiometry of a compound.
- To understand the concept of resonance.
From the outset of our discussion of Lewis Structures, we should emphasize that these things we call Lewis structures do NOT exist in nature. Instead, Lewis structures are a theoretical tool chemists use as a starting point for thinking about how the valence electrons are arranged in a chemical species. Lewis structures give us a tool to figure out if the electrons are bonded between atoms or exist as unshared pairs. When chemistry happens, it is the valence electrons that end up being repositioned by the chemical change.
At the beginning of the 20th century, the American chemist G. N. Lewis (1875–1946) devised a system of symbols—now called Lewis electron dot symbolsA system that can be used to predict the number of bonds formed by most elements in their compounds., often shortened to Lewis dot symbols—that can be used for predicting the number of bonds formed by most elements in their compounds (Figure 6.1(a)). Each Lewis dot symbol consists of the chemical symbol for an element surrounded by dots that represent its valence electrons. Cesium, for example, has the electron configuration [Xe]6s1, which indicates one valence electron outside a closed shell. In the Lewis dot symbol, this single electron is represented as a single dot:
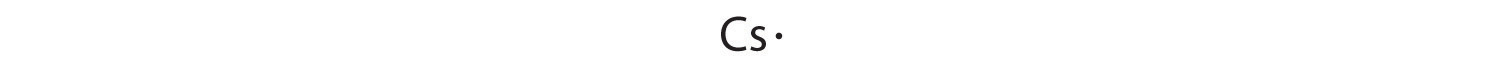
Figure 6.1(a) G. N. Lewis and the Octet Rule
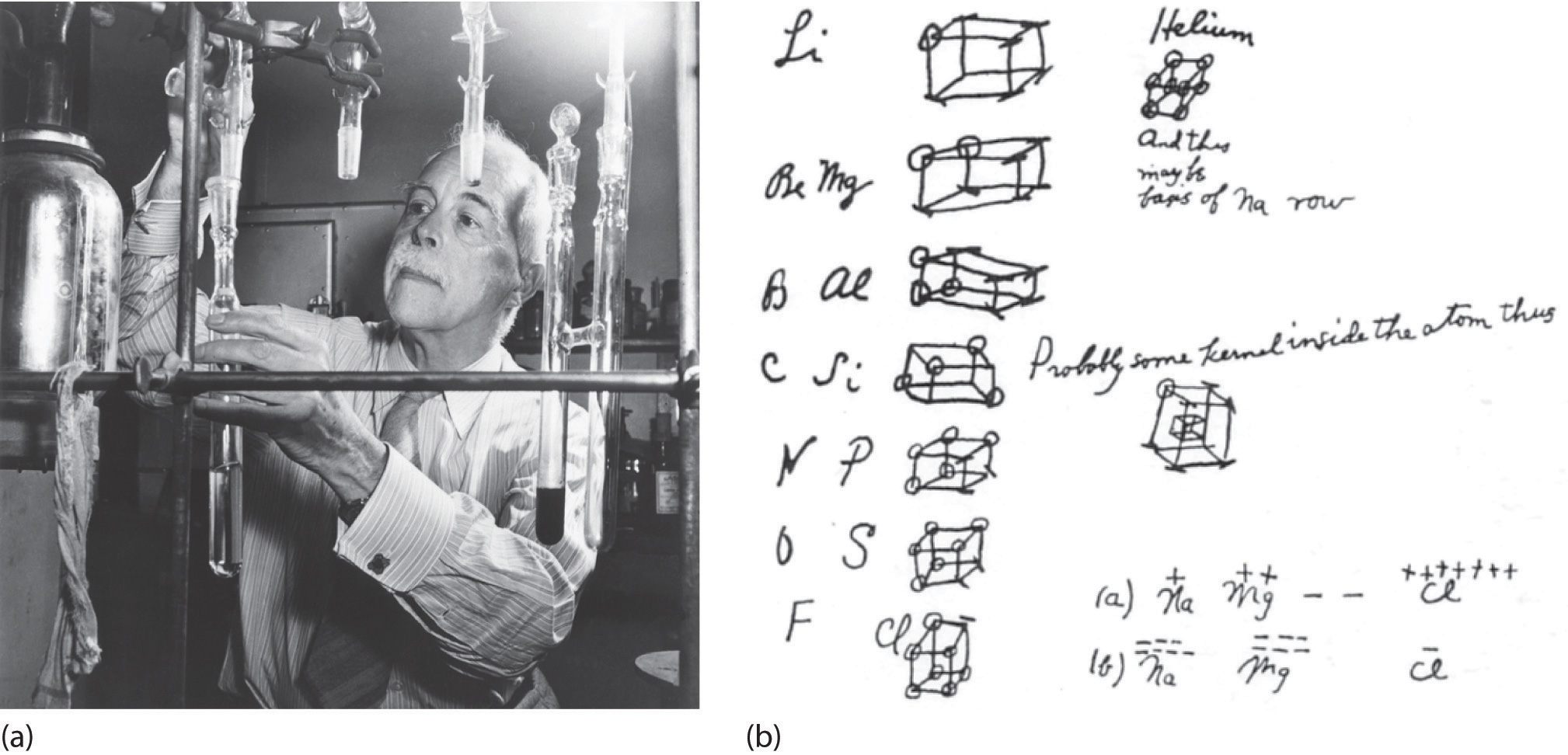
(a) Lewis is working in the laboratory. (b) In Lewis’s original sketch for the octet rule, he initially placed the electrons at the corners of a cube rather than placing them as we do now.
Creating a Lewis Dot Symbol
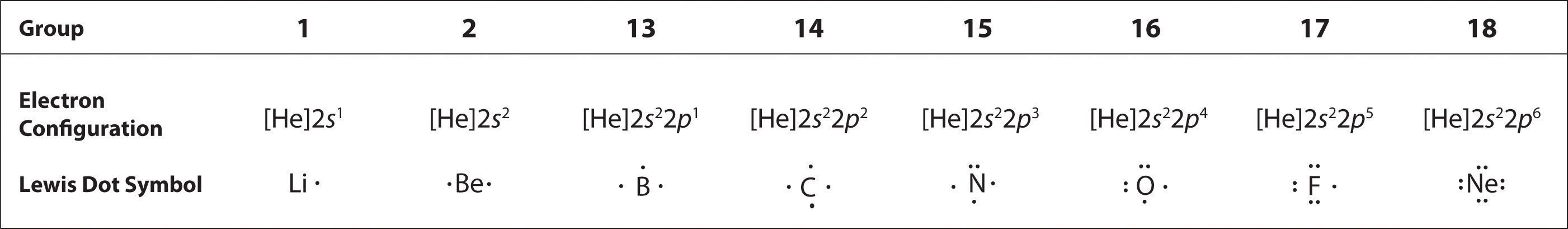
To write an element’s Lewis dot symbol, we place dots representing its valence electrons, one at a time, around the element’s chemical symbol. Up to four dots are placed above, below, to the left, and to the right of the symbol (in any order, as long as elements with four or fewer valence electrons have no more than one dot in each position). The next dots, for elements with more than four valence electrons, are again distributed one at a time, each paired with one of the first four. Fluorine, for example, with the electron configuration [He]2s22p5, has seven valence electrons, so its Lewis dot symbol is constructed as follows:
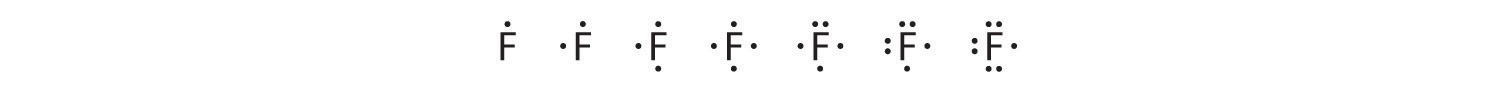
The number of dots in the Lewis dot symbol is the same as the number of valence electrons, which is the same as the last digit of the element’s group number in the periodic table. Lewis dot symbols for the elements in period 2 are given in Figure 6.1(b).
Lewis used the unpaired dots to predict the number of bonds that an element will form in a compound. Consider the symbol for nitrogen in Figure 6.1(b). The Lewis dot symbol explains why nitrogen, with three unpaired valence electrons, tends to form compounds in which it shares the unpaired electrons to form three bonds. Boron, which also has three unpaired valence electrons in its Lewis dot symbol, also tends to form compounds with three bonds, whereas carbon, with four unpaired valence electrons in its Lewis dot symbol, tends to share all of its unpaired valence electrons by forming compounds in which it has four bonds.
Figure 6.1(b) Lewis Dot Symbols for the Elements in Period 2
The Octet Rule
Lewis’s major contribution to bonding theory was to recognize that atoms tend to lose, gain, or share electrons to reach a total of eight valence electrons, called an octet. This so-called octet ruleThe tendency for atoms to lose, gain, or share electrons to reach a total of eight valence electrons. explains the stoichiometry of most compounds in the s and p blocks of the periodic table. We now know from quantum mechanics that the number eight corresponds to one ns and three np valence orbitals, which together can accommodate a total of eight electrons. Remarkably, though, Lewis’s insight was made nearly a decade before Rutherford proposed the nuclear model of the atom. An exception to the octet rule is helium, whose 1s2 electron configuration gives it a full n = 1 shell, and hydrogen, which tends to gain or share its one electron to achieve the electron configuration of helium.
Lewis dot symbols can also be used to represent the ions in ionic compounds. The reaction of cesium with fluorine, for example, to produce the ionic compound CsF can be written as follows:

No dots are shown on Cs+ in the product because cesium has lost its single valence electron to fluorine. The transfer of this electron produces the Cs+ ion, which has the valence electron configuration of Xe, and the F− ion, which has a total of eight valence electrons (an octet) and the Ne electron configuration. This description is consistent with the observation that among the main group elements, ions in simple binary ionic compounds generally have the electron configurations of the nearest noble gas. The charge of each ion is written in the product, and the anion and its electrons are enclosed in brackets. This notation emphasizes that the ions are associated electrostatically; no electrons are shared between the two elements.
Using Lewis Dot Symbols to Describe Covalent Bonding
The valence electron configurations of the constituent atoms of a covalent compound are important factors in determining its structure, stoichiometry, and properties. For example, chlorine, with seven valence electrons, is one electron short of an octet. If two chlorine atoms share their unpaired electrons by making a covalent bond and forming Cl2, they can each complete their valence shell:
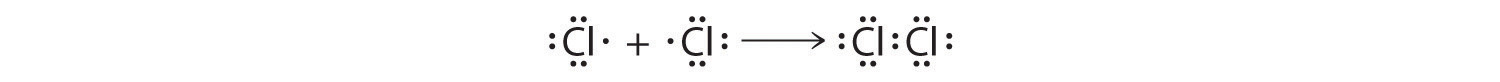
Each chlorine atom now has an octet. The electron pair being shared by the atoms is called a bonding pairA pair of electrons in a Lewis structure that is shared by two atoms, thus forming a covalent bond.; the other three pairs of electrons on each chlorine atom are called lone pairsA pair of electrons in a Lewis structure that is not involved in covalent bonding.. Lone pairs are not involved in covalent bonding. If both electrons in a covalent bond come from the same atom, the bond is called a coordinate covalent bondA covalent bond in which both electrons come from the same atom..
We can illustrate the formation of a water molecule from two hydrogen atoms and an oxygen atom using Lewis dot symbols:
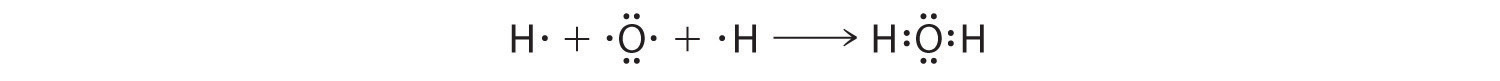
The structure on the right is the Lewis electron structure, or Lewis structure, for H2O. With two bonding pairs and two lone pairs, the oxygen atom has now completed its octet. Moreover, by sharing a bonding pair with oxygen, each hydrogen atom now has a full valence shell of two electrons. Chemists usually indicate a bonding pair by a single line, as shown here for our two examples:

The following procedure can be used to construct Lewis electron structures for more complex molecules and ions:
1. Identify the central atom. Decide which atom is the central atom and which atom(s)is/are surrounding atoms. For our purposes, the central atom is usually of the element for which there is only one atom in the formula. Chemists usually list this central atom first in the chemical formula (as in CCl4 and CO32−, which both have C as the central atom). Hydrogen and the halogens are almost always connected to only one other atom, so they are usually surrounding rather than central.
2. Determine the total number of valence electrons in the molecule or ion. Add together the valence electrons from each atom. (Recall that the number of valence electrons is indicated by the position of the element in the periodic table.) If the species is a polyatomic ion, remember to add or subtract the number of electrons necessary to give the total charge on the ion. For CO32−, for example, we add two electrons to the total because of the −2 charge. So CO32− will have 4 + 18 + 2 = 24 valence electrons.
3. Write the symbol for the central atom and place the symbol for the surrounding atom(s) anywhere around the central atom. Don't worry about perfect positioning of the atoms. You really can't do this wrong as long as you have a central atom surrounded by surrounding atoms. Remember, these are just Lewis structures, merely hypothetical constructs.
4. Use the common bonding patterns in Table 6.1(1) and draw lines between the surrounding atoms and the central atom. Each line represents a shared pair of valence electrons. The patterns in table 6.1(1) (an expanded version of table 4.1(1)) apply to the surrounding atoms. Central atoms are different animals and do NOT necessarily follow these patterns.
Table 6.1(1) Bonding Patterns for Surrounding Atoms
| Atom | Number of Bonds | Number of Unshared Pairs |
|---|---|---|
| H (group 1) | 1 | 0 |
| F (group 17) | 1 | 3 |
| O (group 16) | 2 | 2 |
| N (group 15) | 3 | 1 |
| C (group 14) | 4 | 0 |
In the case of polyatomic anions, put the negative charge on the surrounding atoms. This negatively charged atom will have one fewer bond and one more electron pair than in table 6.1(1). In the case of polyatomic cations, put the positive charge on the central atom. This positively charged atom will have one more bond and one fewer electron pair than listed in table 6.1(1).
5. Put the pairs of dots representing unshared pairs of electrons on the surrounding atoms. Follow the patterns from table 6.1(1), above.
6. Subtract the number of electrons (2 for each line, 1 for each dot) in your drawing from the total number of valence electrons you calculated in step 2. If your Lewis structure is missing some valence electrons, place them on the central atom. C, O, N and F must have 8 electrons around them; otherwise, more than 8 electrons are permitted. It's not at all unusual to see more than 8 electrons around the central atom.
7. Count the formal charge on each atom. This step is a check to evaluate the validity of your proposed Lewis structure. Do this check by considering the formal chargeThe difference between the number of valence electrons in a free atom and the number of electrons assigned to it in a particular Lewis electron structure. on the atoms, which is the difference between the number of valence electrons in the free atom and the number assigned to it in the Lewis electron structure. The formal charge is a way of computing the charge distribution within a Lewis structure; the sum of the formal charges on the atoms within a molecule or an ion must equal the overall charge on the molecule or ion. To count formal charge, subtract from the number of valence electrons contributed by an atom (found in the periodic table), one for each bond, and one for each electron around each atom in your Lewis structure. Always seek to minimize formal charge. Formal charge more positive than +1, or formal charge more negative than -1, is never permitted. Counting formal charge becomes automatic with practice. It is the most important step. Don't leave it out.
Now let's apply this procedure to some particular compounds, beginning with one we have already discussed.
H2O
1. Identify the central atom. There's only one oxygen, so it's the central atom.
2. Determine the total number of valence electrons in the molecule or ion. Each H atom (group 1) has 1 valence electron, and the O atom (group 16) has 6 valence electrons, for a total of 8 valence electrons.
3. Write the symbol for the central atom and place the symbol for the surrounding atom(s) anywhere around the central atom. H O H
4. Use the common bonding patterns in Table 6.1(1) and draw lines between the surrounding atoms and the central atom. Connect the hydrogen atoms to the oxygen with a single bond per table 6.1(1). H-O-H
5. Put the pairs of dots representing unshared pairs of electrons on the surrounding atoms. Table 6.1(1) indicates hydrogen has no unshared pairs of electrons in Lewis structures, so there's nothing to do for this step.
6. Subtract the number of electrons (2 for each line, 1 for each dot) in your drawing from the total number of valence electrons you calculated in step 2. If your Lewis structure is missing some valence electrons, place them on the central atom. We need 8 valence electrons in our Lewis structure. We now have just 4 (each line represents 2 electrons). Adding the remaining 4 electrons to the oxygen (as two lone pairs) gives the following structure:
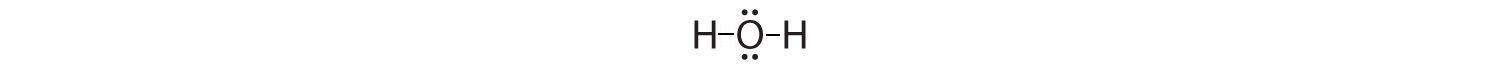
This is the Lewis structure we drew earlier. Because it gives oxygen an octet and each hydrogen two electrons. But for practice let's continue working through the steps by doing step 7.
7. Count the formal charge on each atom For each hydrogen start with 1 valence electron from the periodic table. Subtract one from 1 for the line connecting it to the oxygen. 1-1=0, so no formal charge on the hydrogens. For the oxygen start with 6 valence electrons. Subtract 2 (one for each line) and subtract 4 (for the four dots). 6-2-4=0, so no formal charge on the oxygen. This is a happy Lewis structure.
OCl−
1. Identify the central atom. With only two atoms in the molecule, there is no central atom.
2. Determine the total number of valence electrons in the molecule or ion. Oxygen (group 16) has 6 valence electrons, and chlorine (group 17) has 7 valence electrons; we must add one more for the negative charge on the ion, giving a total of 14 valence electrons.
3. Write the symbol for the central atom and place the symbol for the surrounding atom(s) anywhere around the central atom. O Cl
4. Use the common bonding patterns in Table 6.1(1) and draw lines between the surrounding atoms and the central atom. The hypochlorite ion, ClO-, is going to require a charged atom. As with all ions of oxyacids, put the negative charge on the oxygen. This oxygen will therefore have only one bond as stated in the instructions for step 4. Putting the line representing a pair of electrons between O and Cl gives O-Cl
5. Put the pairs of dots representing unshared pairs of electrons on the surrounding atoms. An uncharged Cl, just like F in table 6.1(1), gets three unshared pairs of electrons. The negatively charged oxygen gets three unshared pairs of electrons. This gives the following structure:
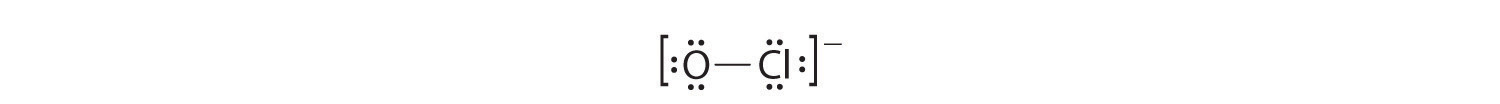
6. Subtract the number of electrons (2 for each line, 1 for each dot) in your drawing from the total number of valence electrons you calculated in step 2. If your Lewis structure is missing some valence electrons, place them on the central atom. We need 14 valence electrons in our Lewis structure. We now have 14 (6 pairs of dots and one line).
7. Count the formal charge on each atom On the oxygen, start with 6 valence electron from the periodic table. Subtract one from 6 for the line connecting it to the chlorine and then subtract one for each of the six dots around the oxygen. 6-1-6=-1, so there is a -1 formal charge on the oxygen atom. For the chlorine start with 7 valence electrons. Subtract one from 7 for the line connecting it to oxygen and subtract 6 (for the six dots). 7-1-6=0, so no formal charge on the chlorine. This is as happy of a Lewis structure as is possible for an ion. Remember ions aren't happy because nature hates charge. The Lewis electron structure is drawn within brackets as is customary for an ion, with the overall charge indicated outside the brackets, and the bonding pair of electrons is indicated by a solid line. OCl− is the hypochlorite ion, the active ingredient in chlorine laundry bleach and swimming pool disinfectant.
CH2O
1. Identify the central atom. This time we have two elements in the formula for which we have only one atom, C and O. There is a hint in the instructions for this first step that leads us to choose the element that is listed first in the formula. So we will make C be the central atom leaving oxygen and hydrogen as surrounding atoms.
2. Determine the total number of valence electrons in the molecule or ion. Each hydrogen atom (group 1) has one valence electron, carbon (group 14) has 4 valence electrons, and oxygen (group 16) has 6 valence electrons, for a total of [(2)(1) + 4 + 6] = 12 valence electrons.
3. Write the symbol for the central atom and place the symbol for the surrounding atom(s) anywhere around the central atom. Placing a bonding pair of electrons between each pair of bonded atoms gives the following:

4. Use the common bonding patterns in Table 6.1(1) and draw lines between the surrounding atoms and the central atom. There's no charge to consider so we use one bond on hydrogen and two bonds (a double bond) on the oxygen giving the following:

5. Put the pairs of dots representing unshared pairs of electrons on the surrounding atoms. Table 6.1(1) indicates no unshared electrons on hydrogen and two unshared pairs on the oxygen.

6. Subtract the number of electrons (2 for each line, 1 for each dot) in your drawing from the total number of valence electrons you calculated in step 2. If your Lewis structure is missing some valence electrons, place them on the central atom. We need 12 valence electrons in our Lewis structure. There are 8 electrons in the 4 lines and 4 dots each representing an unshared electrons. So our figure has 12 valence electrons. There are no electrons left to place on the central atom.
7. Count the formal charge on each atom On the oxygen, start with 6 valence electron from the periodic table. Subtract two from 6 for the lines connecting it to the carbon and then subtract one for each of the four dots around the oxygen. 6-2-4 = 0, so there is no formal charge on the oxygen atom. For either hydrogen, start with 1 valence electron from the periodic table. Subtract one from one for the line. 1-1 = 0, so there is no formal charge on either hydrogen. For the carbon start with 4 valence electrons. Subtract four from 4 for the lines connecting carbon to oxygen and hydrogen. 4-4 = 0, so no formal charge on the carbon. This is a happy Lewis structure. Both the oxygen and the carbon have an octet of electrons. This is the structure of formaldehyde, which is used in embalming fluid.
Example 6.1-1
Write the Lewis electron structure for each species.
- NCl3
- S22−
- NOCl
Given: chemical species
Asked for: Lewis electron structures
Strategy:
Use the seven-step procedure to write the Lewis electron structure for each species.
Solution:
-
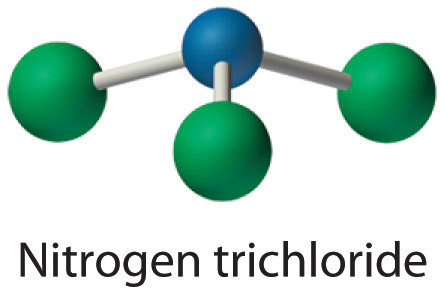
Since there is only one atom of nitrogen in the formula, it is the central atom. The nitrogen atom (group 15) has 5 valence electrons and each chlorine atom (group 17) has 7 valence electrons, for a total of 26 valence electrons. Using Table 6.1(1) we put 1 bond around each chlorine. Then, again using Table 6.1(1) we put three unshared pairs of electrons around each chlorine. We need 26 valence electrons in our structure. We have 24. We need two more electrons, so we put them on the central atom, N. We check formal charge. For Cl, we start with 7 and subtract 1 for the line and 6 for the dots. 7-1-6 = 0. For the formal charge on nitrogen we start with 5 and subtract 3 for the lines and 2 for the dots. 5-3-2 = 0. This is a happy Lewis structure.
Nitrogen trichloride is an unstable oily liquid once used to bleach flour; this use is now prohibited in the United States.
-
In a diatomic molecule or ion, we do not need to worry about a central atom. Each sulfur atom (group 16) contains 6 valence electrons, and we need to add 2 electrons for the -2 charge, giving a total of 14 valence electrons. Using Table 6.1(1), we consider sulfur as analogous to its group-mate oxygen. We need two charges, so each sulfur will have a single bond and three unshared pairs of electrons. This gives us 14 valence electrons in our Lewis structure and 14 is what we needed from step 2. Checking formal charge for either sulfur we start with 6 valence electrons from the periodic table and subtract 1 for the line and 6 for the dots. This gives a formal charge of 6-1-6 = -1 for each sulfur. This is fine since it matches the overall charge on the S22- ion and this is as happy a Lewis structure as we can get for an ion.
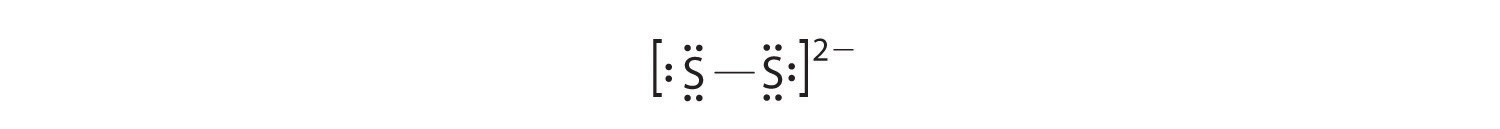
-
Because nitrogen is listed first in the formula, we should assume it is the central atom. The N atom (group 15) has 5 valence electrons, the O atom (group 16) has 6 valence electrons, and the Cl atom (group 17) has 7 valence electrons, giving a total of 18 valence electrons. Table 6.1(1) indicates two bonds on the oxygen (a double bond) and one bond on the chlorine. Table 6.1(1)also indicates two unshared pairs of electrons on oxygen and three unshared pairs of electrons on chlorine. We need 18 valence electrons in our Lewis structure. We have 6 electrons in bonds and 10 electrons as unshared pairs. We need two more electrons, so we put them on the central atom, on the N. Checking formal charge on the oxygen we have 6-2-4 = 0. On the nitrogen we have 5-3-2 = 0. On the chlorine we have 7-1-6 = 0. This is a happy Lewis structure.
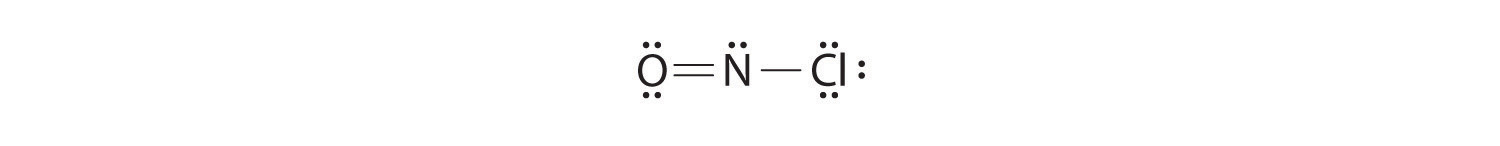
All atoms now have octet configurations. This is the Lewis electron structure of nitrosyl chloride, a highly corrosive, reddish-orange gas.
Exercise
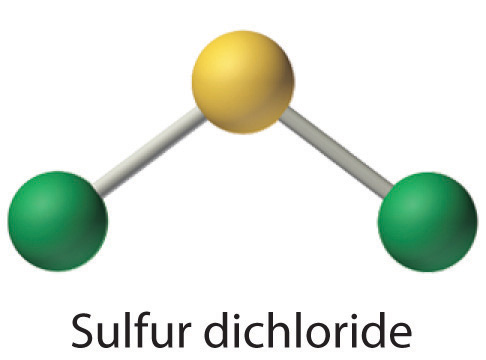
Write Lewis electron structures for CO2 and SCl2, a vile-smelling, unstable red liquid that is used in the manufacture of rubber.
Answer:
Summary
One convenient way to predict the number and basic arrangement of bonds in compounds is by using Lewis electron dot symbols, which consist of the chemical symbol for an element surrounded by dots that represent its valence electrons, grouped into pairs often placed above, below, and to the left and right of the symbol. The structures reflect the fact that the elements in period 2 and beyond tend to gain, lose, or share electrons to reach a total of eight valence electrons in their compounds, the so-called octet rule. In Lewis electron structures, we encounter bonding pairs, which are shared by two atoms, and lone pairs, which are not shared between atoms. If both electrons in a covalent bond come from the same atom, the bond is called a coordinate covalent bond. Lewis structures are an attempt to rationalize why certain stoichiometries are commonly observed for the elements of particular families. Neutral compounds of group 14 elements typically contain four bonds around each atom (a double bond counts as two, a triple bond as three), whereas neutral compounds of group 15 elements typically contain three bonds. In cases where it is possible to write more than one Lewis electron structure with octets around all the nonhydrogen atoms of a compound, the formal charge on each atom in alternative structures must be considered to decide which of the valid structures can be excluded and which is the most reasonable. The formal charge is the difference between the number of valence electrons of the free atom and the number of electrons assigned to it in the compound, where bonding electrons are divided equally between the bonded atoms. The Lewis structure with the lowest formal charges on the atoms is almost always the most stable one.
Conceptual Problems
-
Compare and contrast covalent and ionic compounds with regard to
- volatility.
- melting point.
- electrical conductivity.
- physical appearance.
-
Which atom do you expect to be the central atom in each of the following species?
- SO42−
- NH4+
- BCl3
- SO2Cl2
-
Which atom is the central atom in each of the following species?
- PCl3
- CHCl3
- SO2
- IF3
-
What is the relationship between the number of bonds typically formed by the period 2 elements in groups 14, 15, and 16 and their Lewis electron structures?
-
Although formal charges do not represent actual charges on atoms in molecules or ions, they are still useful. Why?
Numerical Problems
-
Give the electron configuration and the Lewis dot symbol for the following. How many more electrons can each atom accommodate?
- Se
- Kr
- Li
- Sr
- H
-
Give the electron configuration and the Lewis dot symbol for the following. How many more electrons can each atom accommodate?
- Na
- Br
- Ne
- C
- Ga
-
Based on Lewis dot symbols, predict how many bonds gallium, silicon, and selenium will form in their neutral compounds.
-
Determine the total number of valence electrons in the following.
- NO+
- XeF2
- Br2
- CH2Cl2
- NO3−
- H3O+
-
Determine the total number of valence electrons in the following.
- H2S
- OH−
- I2
- CH4
- SO42−
- NH4+
-
Draw Lewis electron structures for the following.
- F2
- SO2
- AlCl4−
- SO32−
- BrCl
- XeF4
- NO+
- PCl3
-
Draw Lewis electron structures for the following.
- Br2
- CH3Br
- SO42−
- O2
- S22−
- BF3
-
Draw Lewis electron structures for CO2, NO2−, SO2, and NO2+. From your diagram, predict which pair(s) of compounds have similar electronic structures.
-
Use Lewis dot symbols to predict whether ICl and NO4− are chemically reasonable formulas.
-
Draw a plausible Lewis electron structure for a compound with the molecular formula Cl3PO.
-
Draw a plausible Lewis electron structure for a compound with the molecular formula CH4O.
-
While reviewing her notes, a student noticed that she had drawn the following structure in her notebook for acetic acid:
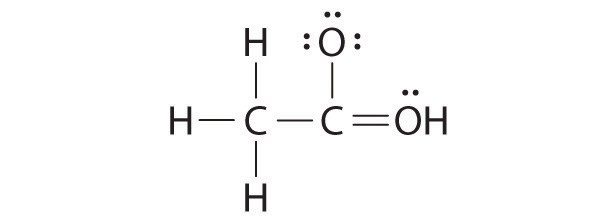
Why is this structure not feasible? Draw an acceptable Lewis structure for acetic acid. Show the formal charges of all nonhydrogen atoms in both the correct and incorrect structures.
-
A student proposed the following Lewis structure shown for acetaldehyde.
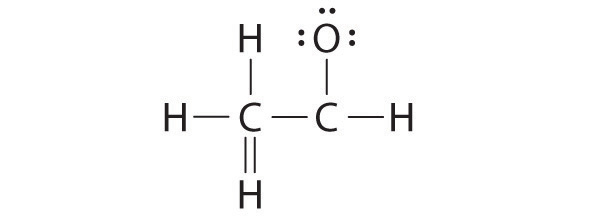
Why is this structure not feasible? Draw an acceptable Lewis structure for acetaldehyde. Show the formal charges of all nonhydrogen atoms in both the correct and incorrect structures.
-
Draw the most likely structure for HCN based on formal charges, showing the formal charge on each atom in your structure. Does this compound have any plausible resonance structures? If so, draw one.
-
Draw the most plausible Lewis structure for NO3−. Does this ion have any other resonance structures? Draw at least one other Lewis structure for the nitrate ion that is not plausible based on formal charges.
-
At least two Lewis structures can be drawn for BCl3. Using arguments based on formal charges, explain why the most likely structure is the one with three B–Cl single bonds.
-
Using arguments based on formal charges, explain why the most feasible Lewis structure for SO42− has two sulfur–oxygen double bonds.
-
At least two distinct Lewis structures can be drawn for N3−. Use arguments based on formal charges to explain why the most likely structure contains a nitrogen–nitrogen double bond.
-
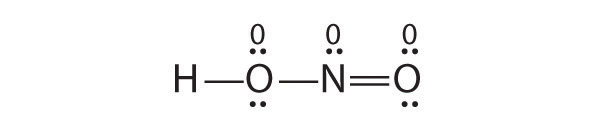
Is H–O–N=O a reasonable structure for the compound HNO2? Justify your answer using Lewis electron dot structures.
-
Is H–O=C–H a reasonable structure for a compound with the formula CH2O? Use Lewis electron dot structures to justify your answer.
-
Explain why the following Lewis structure for SO32− is or is not reasonable.
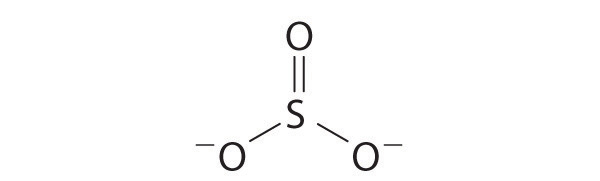
-
Draw all the resonance structures for each ion.
- HSO4−
- HSO3−
Answers
-
-
[Ar]4s23d104p4
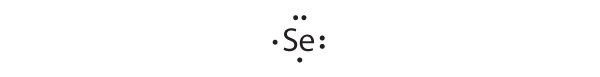
Selenium can accommodate two more electrons, giving the Se2− ion.
-
[Ar]4s23d104p6
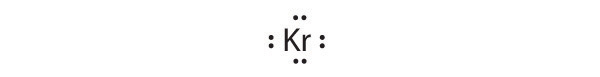
Krypton has a closed shell electron configuration, so it cannot accommodate any additional electrons.
-
1s22s1
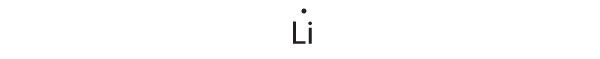
Lithium can accommodate one additional electron in its 2s orbital, giving the Li− ion.
-
[Kr]5s2
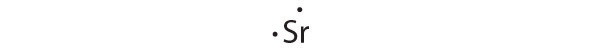
Strontium has a filled 5s subshell, and additional electrons would have to be placed in an orbital with a higher energy. Thus strontium has no tendency to accept an additional electron.
-
1s1

Hydrogen can accommodate one additional electron in its 1s orbital, giving the H− ion.
-
-
-
-
-
- 8
- 8
- 14
- 8
- 32
- 8
-
-
-
-
-
-
-
The only structure that gives both oxygen and carbon an octet of electrons is the following:
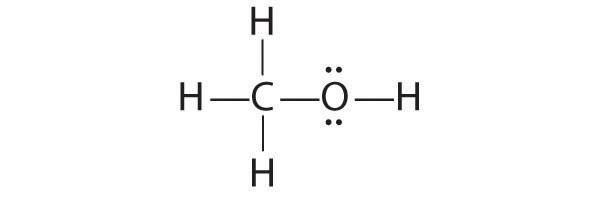
-
-
The student’s proposed structure has two flaws: the hydrogen atom with the double bond has four valence electrons (H can only accommodate two electrons), and the carbon bound to oxygen only has six valence electrons (it should have an octet). An acceptable Lewis structure is

The formal charges on the correct and incorrect structures are as follows:
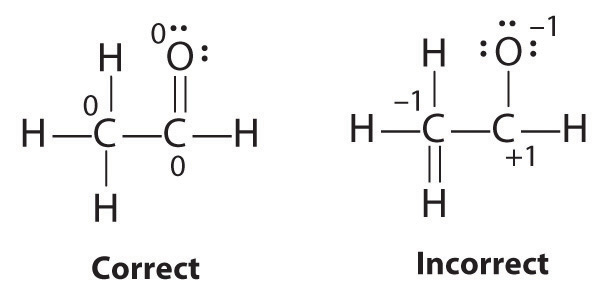
-
-
The most plausible Lewis structure for NO3− is:
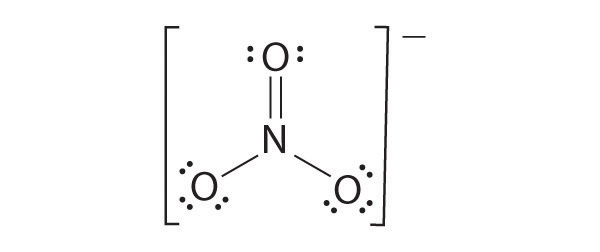
There are three equivalent resonance structures for nitrate (only one is shown), in which nitrogen is doubly bonded to one of the three oxygens. In each resonance structure, the formal charge of N is +1; for each singly bonded O, it is −1; and for the doubly bonded oxygen, it is 0.
The following is an example of a Lewis structure that is not plausible:

This structure nitrogen has six bonds (nitrogen can form only four bonds) and a formal charge of –1.
-
-
With four S–O single bonds, each oxygen in SO42− has a formal charge of −1, and the central sulfur has a formal charge of +2. With two S=O double bonds, only two oxygens have a formal charge of –1, and sulfur has a formal charge of zero. Lewis structures that minimize formal charges tend to be lowest in energy, making the Lewis structure with two S=O double bonds the most probable.
-
-
Yes. This is a reasonable Lewis structure, because the formal charge on all atoms is zero, and each atom (except H) has an octet of electrons.
-
-
-