This is "Unit 5", section 5.1 from the book General Chemistry (v. 1.0).
5.1 Naming Ionic Compounds When The Metal Has More Than One Possible Charge
Learning Objective
- To name ionic compounds.
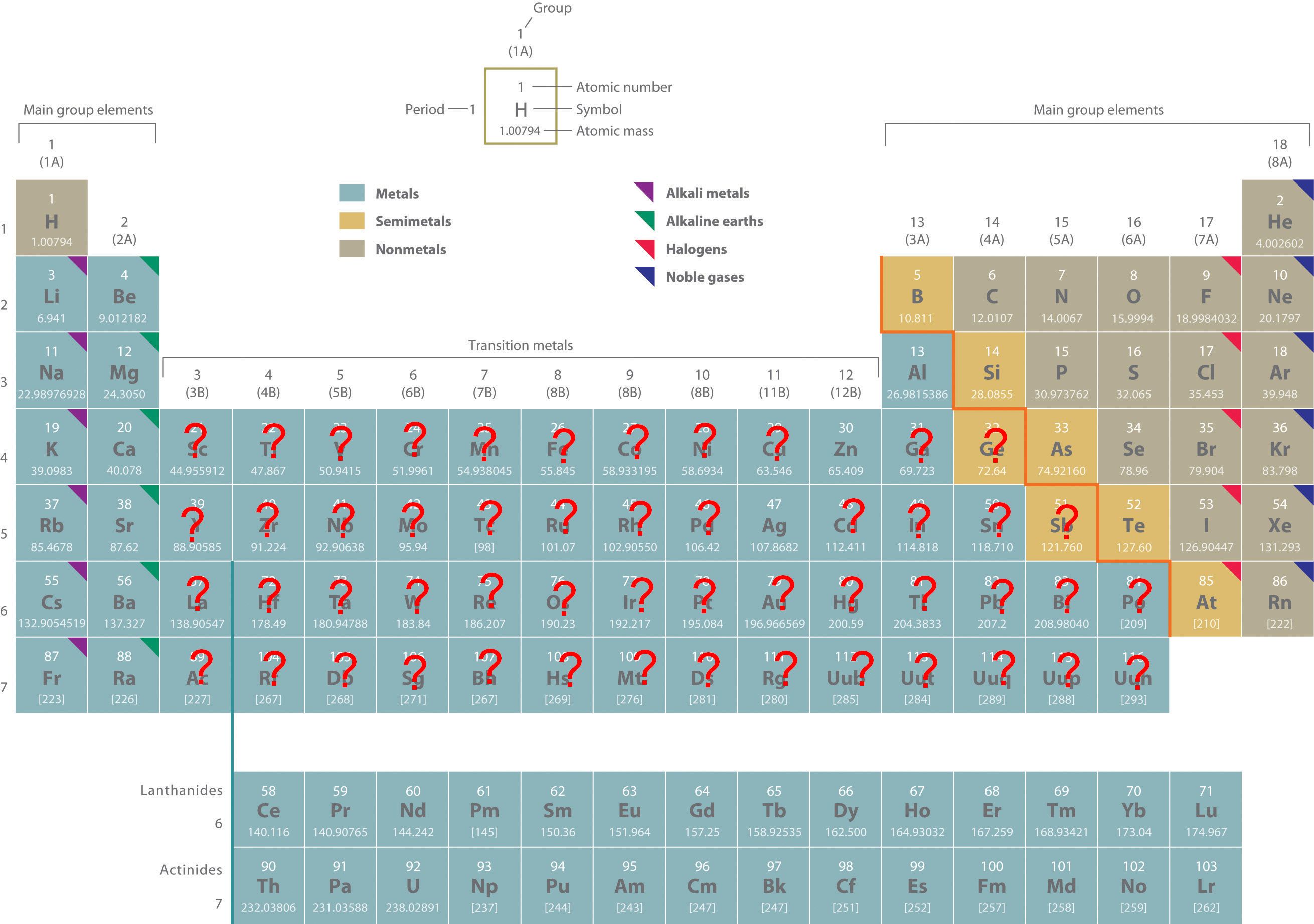
In this unit we are still learning to write names and formulas of compounds. Yes, you already know a great deal about names and formulas of compounds. You know how to write names and formulas for binary molecular compounds. You know how to write names and formulas for many binary ionic compounds. But, like always it seems, there are complications. In this section we will tackle binary ionic compounds that have a metal with more than one possible charge. These metals are tagged with question marks in Figure 5.1(a)
Figure 5.1(a) Periodic Table
We will be using exactly the same strategies we learned for naming binary ionic compounds in unit 4. When we encounter metals with multiple charges, however, we must include a roman numeral in the name indicating the charge. Our modified procedure for naming such compounds is outlined in Figure 5.1(b) "Naming an Ionic Compound" and uses the following steps:
Figure 5.1(b) Naming an Ionic Compound
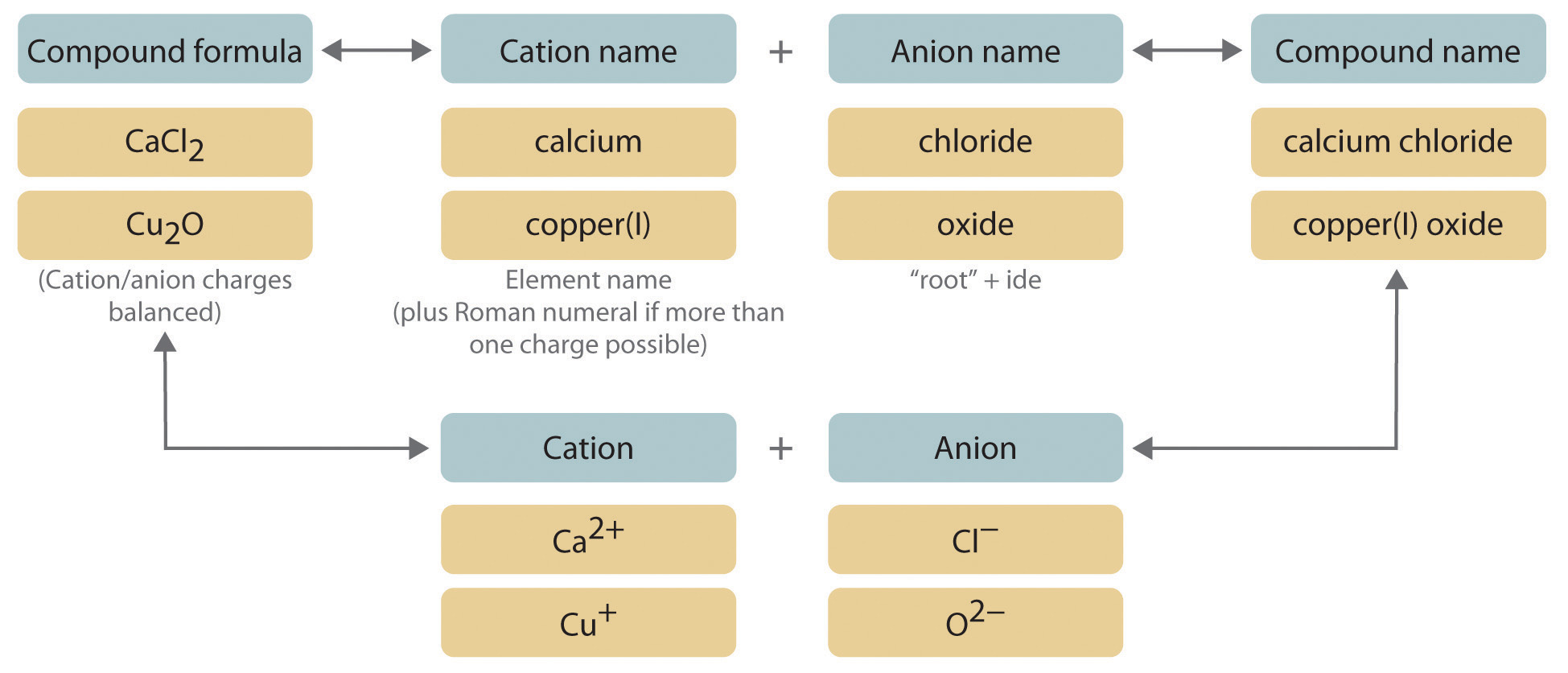
- Place the ions in their proper order: cation and then anion.
Name the cation.
- Metals that form only one cation.
Metals that form more than one cation. As shown in Figure 5.1(a) , many metals can form more than one cation. This behavior is observed for most transition metals, many actinides, and the heaviest elements of groups 13–15. In such cases, the positive charge on the metal is indicated by a roman numeral in parentheses immediately following the name of the metal. Thus Cu+ is copper(I) (read as “copper one”), Fe2+ is iron(II), Fe3+ is iron(III), Sn2+ is tin(II), and Sn4+ is tin(IV).
An older system of nomenclature for such cations is still widely used, however. The name of the cation with the higher charge is formed from the root of the element’s Latin name with the suffix -ic attached, and the name of the cation with the lower charge has the same root with the suffix -ous. The names of Fe3+, Fe2+, Sn4+, and Sn2+ are therefore ferric, ferrous, stannic, and stannous, respectively. This text uses the systematic names with roman numerals. Though it won't be a topic for examination in the course, you may want to be able to recognize these common names because they are still often used. For example, on the label of your dentist’s fluoride rinse, the compound chemists call tin(II) fluoride is usually listed as stannous fluoride.
Some examples of metals that form more than one cation are in Table 5.1(1) along with the names of the ions. Note that the simple Hg+ cation does not occur in chemical compounds. Instead, all compounds of mercury(I) contain a dimeric cation, Hg22+, in which the two Hg atoms are bonded together.
Table 5.1(1) Common Cations of Metals That Form More Than One Ion
Cation Systematic Name Common Name Cation Systematic Name Common Name * Not widely used. †The isolated mercury(I) ion exists only as the gaseous ion. Cr2+ chromium(II) chromous Cu2+ copper(II) cupric Cr3+ chromium(III) chromic Cu+ copper(I) cuprous Mn2+ manganese(II) manganous* Hg2+ mercury(II) mercuric Mn3+ manganese(III) manganic* Hg22+ mercury(I) mercurous† Fe2+ iron(II) ferrous Sn4+ tin(IV) stannic Fe3+ iron(III) ferric Sn2+ tin(II) stannous Co2+ cobalt(II) cobaltous* Pb4+ lead(IV) plumbic* Co3+ cobalt(III) cobaltic* Pb2+ lead(II) plumbous*
Name the anion.
Write the name of the compound as the name of the cation followed by the name of the anion.
Note the Pattern
Most transition metals and the heaviest elements of groups 13–15 can form more than one cation.
Example 5.1-1
Write the systematic name (and the common name if applicable) for each ionic compound.
- FeCl3
- MnO2
- Cr2S3
- CuO
Given: formula
Asked for: name
Strategy:
A If only one charge is possible for the cation, give its name. If the cation can have more than one charge (Table 5.1(1) ), specify the charge using roman numerals.
B Name the anion.
C Beginning with the cation, write the name of the compound.
Solution:
- A B We have to figure out the charge on iron from the formula of the compound. We know that the Cl in the formula is representing chloride ion. We know the charge on chloride ion is 1- and there are three chlorides in the formula; therefore, since all compounds are neutral, we know the charge of iron is 3+ and this is the iron(III) cation. C Because we list the cation first, the name of this compound is iron(III) chloride.
- A B Again, since manganese is one of the metals you can't know the charge for by simply looking at the periodic table, you'll need to figure out the charge on manganese. You know the anion is the oxide ion and it's charge is 2-. Since there are two oxide anions in the compound and the compound is neutral, the charge on manganse must be 4+. The name of the cation is manganse(IV). C Because we list the cation first, the name of this compound is magnesse(IV) oxide.
- A B Recognizing we don't know the charge of chromium by looking at the periodic table we look first to the anion. The anion is the sulfide ion and its charge from the location in the periodic table is 2-. There are three sulfide ions for a total of 6- charge in the compound. There are two chromium cations in the formula and we need a total of 6+ charge for a neutral compound. Each chromium must therefore be 3+. This is the Cr(III) cation. C The compound is therefore chromium(III) sulfide.
- A B The cation is a transition metal that often forms more than one cation. We must therefore specify the positive charge on the cation in the name: copper(II) or, according to the older system, cupric. The anion is oxide. C The name of this compound is copper(II) oxide or, in the older system, cupric oxide. We will be "winning" elemental copper from a bottle labeled cupric oxide in the lab for this course.
Exercise
Write the systematic name (and the common name if applicable) for each ionic compound.
- CuCl
- MgCl2
- Fe2O3
Answer:
- copper(I) chloride (or cuprous chloride)
- magnesium chloride (NO ROMAN NUMERAL)
- iron(III) oxide (or ferric oxide)
Example 5.1-2
Write the formula for each compound.
- gold(V) fluoride
- tin(II) chloride
- chromium(III) oxide
Given: systematic name
Asked for: formula
Strategy:
A Identify the cation. The charge of the cation is explicitly given in the name as a roman numeral.
B Identify the anion. The charge is found by its location in the periodic table.
C Beginning with the cation, write the compound's formula and then determine the number of cations and anions needed to achieve electrical neutrality. Alternatively you may also use the "crossing charge technique" outlined in the previous section, Section 4.4.
Solution:
- A The name indicates the charge of gold is 5+. B Fluoride's position in the periodic table indicates its charge is 1-. C Five F− ions are needed to balance the positive charge on Au5+, to give the formula AuF5.
- A The name of the compound indicates the charge of tin is 2+. B Chloride is Cl-. CTo balance the electrical charges, we need two Cl- anions to balance one Sn2+cation, giving SnCl2.
- A The roman numeral tells us that the positive charge in this case is +3, so the cation is Cr3+. B Oxide is O2−.C Thus two cations (Cr3+) and three anions (O2−) are required to give an electrically neutral compound, Cr2O3. This compound is a common green pigment that has many uses, including camouflage coatings.

Cr2O3. Chromium(III) oxide (Cr2O3) is a common pigment in dark green paints, such as camouflage paint. Image Credit: Tubes are from By Дар Ветер (Own work) [CC BY-SA 3.0 (http://creativecommons.org/licenses/by-sa/3.0)], via Wikimedia Commons. Top Right Inset (Powder) is from By FK1954 (Own work) [Public domain], via Wikimedia Commons. Bottom Right Inset: Nova Color Cerulean Blue Hue and Chromium Oxide Green applied with a palette knife by Melissa Dinwiddie https://creativecommons.org/licenses/by-sa/2.0/ via Flickr
Exercise
Write the formula for each compound.
- tungsten(IV) sulfide
- titanium(III) nitride
- mercury(II) selenide
Answer:
- WS2
- TiN
- HgSe
Summary
Ionic compounds are named according to systematic procedures, although common names are widely used. Systematic nomenclature enables us to write the structure of any compound from its name and vice versa. Ionic compounds are named by writing the cation first, followed by the anion. If a metal can form cations with more than one charge, the charge is indicated by roman numerals in parentheses following the name of the metal.
Key Takeaway
- There is a systematic method used to name ionic compounds.
Conceptual Problems
-
Name each compound.
- MgBr2
- MnBr2
- CaO
- Co2O3
- PtCl4
-
Name each compound.
- FeF3
- Zn3N2
- PtCl2
- CuBr
- CrF6
Answers
-
- magnesium bromide
- mangansese(II) bromide
- calcium oxide
- cobalt(III) oxide
- platinum(IV) chloride
-
- iron(III) fluoride
- zinc nitride
- platinum(II) chloride
- copper(I) bromide
- chromium(VI) fluoride
Numerical Problems
-
For each ionic compound, name the cation and the anion and give the charge on each ion.
- PbCl2
- SbCl5
- V2O5
- CuF
- MoS2
-
Write the formula for each compound.
- titanium(IV) chloride
- lead(II) iodide
- iron(II) oxide
- lead(IV) oxide
- nickel(II) bromide
Answers
-
- lead(II) cation, 2+; chloride anion, 1-
- antimony(V) cation, 5+; chloride anion, 1-
- vanadium(V) cation, 5+; oxide anion, 2-
- copper(I) cation, 1+; fluoride anion, 1-
- molybdenum(IV) cation, 4+; sulfide anion, 2-
-
- TiCl4
- PbI2
- FeO
- PbO2
- NiBr2





